Functional reconstitution of COPI coat assembly and disassembly using chemically defined components - PubMed (original) (raw)
Functional reconstitution of COPI coat assembly and disassembly using chemically defined components
Constanze Reinhard et al. Proc Natl Acad Sci U S A. 2003.
Abstract
Coat protein I (COPI)-coated transport vesicles mediate protein and lipid transport in the early secretory pathway. The basic machinery required for the formation of these transport intermediates has been elucidated based on the reconstitution of COPI-coated vesicle formation from chemically defined liposomes. In this experimental system, the coat components coatomer and GTP-bound ADP-ribosylation factor (ARF), as well as p23 as a membrane-bound receptor for COPI coat proteins, were shown to be both necessary and sufficient to promote COPI-coated vesicle formation. Based on biochemical and ultrastructural analyses, we now demonstrate that the catalytic domain of ARF-GTPase-activating protein (GAP) alone is sufficient to initiate uncoating of liposome-derived COPI-coated vesicles. By contrast, ARF-GAP activity is not required for COPI coat assembly and, therefore, does not seem to represent an essential coat component of COPI vesicles as suggested recently [Yang, J. S., Lee, S. Y., Gao, M., Bourgoin, S., Randazzo, P. A., et al. (2002) J. Cell Biol. 159, 69-78]. Thus, a complete round of COPI coat assembly and disassembly has been reconstituted with purified components defining the core machinery of COPI vesicle biogenesis.
Figures
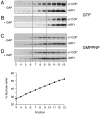
Fig. 1.
COPI budding from liposomes in the presence or absence of the catalytic domain of ARF-GAP. COPI budding assays using Golgi-like liposomes containing p23-lipopeptide, recombinant ARF1, and coatomer were performed in the presence (B and D) or absence (A and C) of the catalytic domain of ARF-GAP. The experiments shown in A and_B_ were performed in the presence of GTP; those shown in _C_and D are in the presence of GMPPNP. After incubation for 30 min at 37°C, samples were separated by flotation in sucrose density gradients. Fractions 3–13 (see Materials and Methods for details) were separated on SDS gels, followed by immunodetection of β′-COP and ARF1 based on Western blotting. Liposome-derived COPI-coated vesicles migrate in fractions 7–9 corresponding to a sucrose density of ≈40% (wt/wt).
Fig. 2.
Postincubation of COPI budding samples with ARF-GAP activity. Liposome-derived COPI-coated vesicles were generated in the presence of GTP as described in the legend of Fig. 1. Thereafter, the sample was split, with one half being mock-treated (A) and the other half incubated with the catalytic domain of ARF-GAP (B). Samples were analyzed by flotation in sucrose density gradients and SDS/PAGE–Western blotting as described in the legend to Fig. 1. For details, see Materials and Methods.
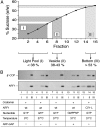
Fig. 3.
Uncoating of liposome-derived COPI vesicles depends on ARF1-GTP hydrolysis triggered by the catalytic domain of ARF-GAP. Liposome-derived COPI-coated vesicles were generated as described in the legend of Fig. 1. As indicated, a number of parameters such as the use of GTP versus GMPPNP, ARF1 wild-type versus ARF-Q71L, and temperature (37°C versus 4°C) were varied. Samples were separated by flotation in sucrose density gradients as described in the legend to Fig. 1. Fractions were collected as pool I (donor liposomes), pool II (coated vesicles), and pool III (load) as indicated in A. For each experimental condition shown in_B_, 2.5% of pool I and II as well as 0.5% of pool III were analyzed by SDS/PAGE and Western blotting using anti-β′-COP and anti-ARF1 antibodies as described in the legend to Fig. 1.
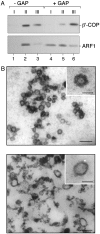
Fig. 4.
Ultrastructural analysis of ARF-GAP-induced uncoating of COPI-coated vesicles. Liposome-derived COPI-coated vesicles were generated in the presence of GTP as described in the legend of Fig. 1. (A) After isolation by flotation in sucrose density gradients the coated vesicle fraction (pool II, see legend of Fig. 3) was split, with one half being mock-treated and the other half incubated with the catalytic domain of ARF-GAP. Thereafter, coated vesicles were reisolated based on a second flotation gradient that was again fractionated into three pools as described in the legend to Fig. 3. For each experimental condition shown in A, 25% of pool I and II as well as 5% of pool III were analyzed by SDS/PAGE and Western blotting using anti-β′-COP and anti-ARF1 antibodies as described in the legend to Fig. 1. (B) COPI vesicles were generated in the presence of GTP and isolated as described in the legend to Fig. 3. After incubation in the presence or absence of the catalytic domain of ARF-GAP, samples were processed for electron microscopy as described under_Materials and Methods_. The bars in the main panels correspond to 100 nm; the bars in the Insets correspond to 50 nm.
Similar articles
- Lipid packing sensed by ArfGAP1 couples COPI coat disassembly to membrane bilayer curvature.
Bigay J, Gounon P, Robineau S, Antonny B. Bigay J, et al. Nature. 2003 Dec 4;426(6966):563-6. doi: 10.1038/nature02108. Nature. 2003. PMID: 14654841 - Membrane curvature and the control of GTP hydrolysis in Arf1 during COPI vesicle formation.
Antonny B, Bigay J, Casella JF, Drin G, Mesmin B, Gounon P. Antonny B, et al. Biochem Soc Trans. 2005 Aug;33(Pt 4):619-22. doi: 10.1042/BST0330619. Biochem Soc Trans. 2005. PMID: 16042557 Review. - ARFGAP1 plays a central role in coupling COPI cargo sorting with vesicle formation.
Lee SY, Yang JS, Hong W, Premont RT, Hsu VW. Lee SY, et al. J Cell Biol. 2005 Jan 17;168(2):281-90. doi: 10.1083/jcb.200404008. J Cell Biol. 2005. PMID: 15657398 Free PMC article. - ArfGAP1 activity and COPI vesicle biogenesis.
Beck R, Adolf F, Weimer C, Bruegger B, Wieland FT. Beck R, et al. Traffic. 2009 Mar;10(3):307-15. doi: 10.1111/j.1600-0854.2008.00865.x. Epub 2008 Dec 4. Traffic. 2009. PMID: 19055691 - The COPI system: molecular mechanisms and function.
Beck R, Rawet M, Wieland FT, Cassel D. Beck R, et al. FEBS Lett. 2009 Sep 3;583(17):2701-9. doi: 10.1016/j.febslet.2009.07.032. Epub 2009 Jul 22. FEBS Lett. 2009. PMID: 19631211 Review.
Cited by
- Dominant ARF3 variants disrupt Golgi integrity and cause a neurodevelopmental disorder recapitulated in zebrafish.
Fasano G, Muto V, Radio FC, Venditti M, Mosaddeghzadeh N, Coppola S, Paradisi G, Zara E, Bazgir F, Ziegler A, Chillemi G, Bertuccini L, Tinari A, Vetro A, Pantaleoni F, Pizzi S, Conti LA, Petrini S, Bruselles A, Prandi IG, Mancini C, Chandramouli B, Barth M, Bris C, Milani D, Selicorni A, Macchiaiolo M, Gonfiantini MV, Bartuli A, Mariani R, Curry CJ, Guerrini R, Slavotinek A, Iascone M, Dallapiccola B, Ahmadian MR, Lauri A, Tartaglia M. Fasano G, et al. Nat Commun. 2022 Nov 11;13(1):6841. doi: 10.1038/s41467-022-34354-x. Nat Commun. 2022. PMID: 36369169 Free PMC article. - ArfGAP1 promotes COPI vesicle formation by facilitating coatomer polymerization.
Shiba Y, Luo R, Hinshaw JE, Szul T, Hayashi R, Sztul E, Nagashima K, Baxa U, Randazzo PA. Shiba Y, et al. Cell Logist. 2011 Jul-Dec;1(4):139-154. doi: 10.4161/cl.1.4.18896. Epub 2011 Jul 1. Cell Logist. 2011. PMID: 22279613 Free PMC article. - The Gcs1 Arf-GAP mediates Snc1,2 v-SNARE retrieval to the Golgi in yeast.
Robinson M, Poon PP, Schindler C, Murray LE, Kama R, Gabriely G, Singer RA, Spang A, Johnston GC, Gerst JE. Robinson M, et al. Mol Biol Cell. 2006 Apr;17(4):1845-58. doi: 10.1091/mbc.e05-09-0832. Epub 2006 Feb 1. Mol Biol Cell. 2006. PMID: 16452633 Free PMC article. - Three homologous ArfGAPs participate in coat protein I-mediated transport.
Saitoh A, Shin HW, Yamada A, Waguri S, Nakayama K. Saitoh A, et al. J Biol Chem. 2009 May 15;284(20):13948-13957. doi: 10.1074/jbc.M900749200. Epub 2009 Mar 19. J Biol Chem. 2009. PMID: 19299515 Free PMC article. - The ArfGAP Glo3 is required for the generation of COPI vesicles.
Lewis SM, Poon PP, Singer RA, Johnston GC, Spang A. Lewis SM, et al. Mol Biol Cell. 2004 Sep;15(9):4064-72. doi: 10.1091/mbc.e04-04-0316. Epub 2004 Jul 14. Mol Biol Cell. 2004. PMID: 15254269 Free PMC article.
References
- Kirchhausen, T. (2000) Nat. Rev. Mol. Cell Biol. 1 187-198. - PubMed
- Nickel, W., Brügger, B. & Wieland, F. T. (2002) J. Cell Sci. 115 3235-3240. - PubMed
- Rothman, J. E. (1994) Nature 372 55-63. - PubMed
- Schekman, R. & Orci, L. (1996) Science 271 1526-1533. - PubMed
- Schmid, S. L. (1997) Annu. Rev. Biochem. 66 511-548. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous