Memory CD4+ T cells do not induce graft-versus-host disease - PubMed (original) (raw)
Memory CD4+ T cells do not induce graft-versus-host disease
Britt E Anderson et al. J Clin Invest. 2003 Jul.
Abstract
Graft-versus-host disease (GVHD) remains a major cause of morbidity and mortality in allogeneic stem cell transplantation (alloSCT). Donor T cells that accompany stem cell grafts cause GVHD by attacking recipient tissues; therefore, all patients receive GVHD prophylaxis by depletion of T cells from the allograft or through immunosuppressant drugs. In addition to providing a graft-versus-leukemia effect, donor T cells are critical for reconstituting T cell-mediated immunity. Ideally, immunity to infectious agents would be transferred from donor to host without GVHD. Most donors have been exposed to common pathogens and have an increased precursor frequency of memory T cells against pathogenic antigens. We therefore asked whether memory CD62L-CD44+ CD4+ T cells would induce less GVHD than unfractionated or naive CD4+ T cells. Strikingly, we found that memory CD4 cells induced neither clinical nor histologic GVHD. This effect was not due to the increased number of CD4+CD25+ regulatory T cells found in the CD62L-CD44+ fraction because memory T cells depletion of these cells did not cause GVHD. Memory CD4 cells engrafted and responded to antigen both in vivo and in vitro. If these murine results are applicable to human alloSCT, selective administration of memory T cells could greatly improve post-transplant immune reconstitution.
Figures
Figure 1
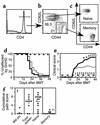
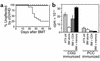
Memory CD4+ T cells do not cause GVHD. Naive and memory T cells were purified as described in Methods. After gating on CD4+ T cells (a), cells were sorted into CD62L+CD44– naive and CD62L–CD44+ memory fractions (b). Reanalyses of sorted populations are shown in (c). BALB/c mice were lethally irradiated and reconstituted with 8 × 106 B10.D2 T cell–depleted BM alone (thin dashed line, n = 9) or with 107 B10.D2 total spleen cells (thin solid line, n = 25), 106 naive T cells (thick solid line, n = 20), or 106 memory T cells (thick dashed line, n = 10). Data are combined from two independent experiments. GVHD incidence and mean clinical score are shown in d and e. Statistical comparisons are as follows: (d). P < 0.0001 for GVHD incidence in recipients of memory CD4 versus spleen cells or naive CD4 cells. (e) For clinical score, *P < 0.05 (time points 1–3) and ‡P < 0.01 (time points 4–6) for recipients of naive versus total spleen cells; †P < 0.05. §P < 0.001 (time points 2–10) for recipients of memory versus total spleen cells. P < 0.0001 for recipients of memory versus naive cells at all time points. BM control mice and BM plus memory cell groups did not get GVHD, but the clinical score lines were offset for clarity. Pathology scores from representative mice are shown in (f). Mean scores are indicated by horizontal bars. ††P < 0.005 and P < 0.0004 for recipients of memory versus total (unfractionated) spleen cells and memory versus naive CD4 cells, respectively.
Figure 2
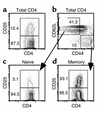
CD25 expression on naive and memory CD4 subsets. B10.D2 spleen cells enriched for CD4 cells as described in Methods were stained with mAb’s against CD4, CD25, CD62L, and CD44. We found that 12.4% of CD4 cells were CD25+ (a). We gated on CD62L+CD44– naive and CD62L–CD44+ memory CD4 cells (b) and analyzed their expression of CD25 (c and d). Note that 33.1% of cells with a memory phenotype express CD25 versus 5.1% of cells with a naive phenotype.
Figure 3
FACS-sorted memory CD25– T cells do not cause GVHD. Donor B10.D2 spleen cells were enriched for CD4+ T cells using BioMag separation, then stained with mAb’s for CD4, CD25, CD62L, and CD44. After gating on CD4+CD25– cells (a), T cells were sorted on the basis of CD62L and CD44 expression (b). Reanalyses of sorted populations are shown in (c) (CD44 versus CD62L) and (d) (CD4 versus CD25). BALB/c mice were lethally irradiated and reconstituted with 8 × 106 B10.D2 T cell–depleted BM alone (thin dashed line, n = 1) or with 2 × 106 B10.D2 unfractionated CD4+ T cells (thin solid line, n = 4), 106 purified naive CD4+CD25– T cells (thick solid line, n = 9), or 106 memory CD4+CD25– T cells (thick dashed line, n = 3). Incidence of GVHD is shown in (e). P < 0.0082 and P < 0.0005 comparing GVHD incidence in recipients of CD25– memory CD4 versus unfractionated and CD25– naive CD4 cells, respectively. Average clinical disease score for affected mice (f). *P < 0.05 (time points 1–3) and for recipients of CD25– naive cells versus unfractionated CD4 cells. P < 0.02 for all comparisons between recipients of CD25– memory and naive cells. Pathology scoring from representative mice (g). ††P < 0.0034 and P < 0.017 for recipients of memory versus total and naive CD4+ T cells, respectively. **P < 0.016 for recipients of naive versus total CD4+ T cells.
Figure 4
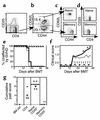
AutoMACS- and FACS-sorted CD25-depleted memory T cells do not cause GVHD. Donor B10.D2 spleen cells enriched for CD4+ T cells using BioMag beads were stained with biotinylated anti-CD62L and anti-CD25 mAb’s, followed by staining with SA-beads. Cells were separated into CD25–CD62L– (negative [neg] fraction) and CD25+CD62L+ (positive [pos] fraction) cells using an AutoMACS. Phenotype of presort CD4+ T cells is shown in (a). Phenotype of CD25–CD62L– negative fraction (memory cells) is shown in (b). CD25+CD62L+ cells (positive fraction) were sorted on a FACStar cell sorter to purify CD25– (c) and CD62L+CD44– cells (d). Reanalysis of the sorted population is not available. BALB/c mice were lethally irradiated and reconstituted with 8 × 106 B10.D2 T cell–depleted BM alone (thin dashed line, n = 5) or with 1.5 × 106 unfractionated B10.D2 CD4+ T cells (thin solid line, n = 10), 2.5 × 105 CD4+CD25– naive T cells (thick solid line, n = 5), or 106 CD4+CD25– memory T cells (thick dashed line, n = 4). Incidence of GVHD (e). P < 0.0002 and P < 0.003 for difference between recipients of CD25– memory and total CD4 and CD25– naive CD4 cells, respectively. Average clinical disease score for mice affected with GVHD (f). *P < 0.02 (all time points) for CD25– memory versus total CD4. P < 0.01 on days 19–43 after transplant for recipients of CD25– memory versus naive CD4 cells. Pathology scoring from representative mice (g). #P < 0.007 and P < 0.014 for recipients of CD25– memory versus total and CD25– naive CD4 cells, respectively.
Figure 5
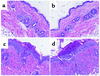
Representative histology. Representative skin histology from BALB/c recipients of B10.D2 T cell–depleted BM alone (a), with memory cells (b), unfractionated CD4 cells (c), or naive CD4 cells (d). Note thickening of keratinocyte layer, interface dermatitis, and ulcerations (c and d) not present in a and b.
Figure 6
Donor memory cells engraft and respond to antigenic challenge. B10.D2 mice were immunized intraperitoneally with CGG in CFA and used 3 weeks later as CD4+ cell and BM donors. ATX BALB/c mice were irradiated and reconstituted with 8 × 106 T cell–depleted BM cells with no CD4 cells (thin dashed line, n = 9), 1.5 × 106 unfractionated CD4 cells (thin solid line, n = 17), or 106 CD4+CD25– memory cells (thick line, n = 14). Incidence of GVHD (a). P < 0.001 for GVHD incidence in recipients of CD25– memory cells versus total CD4 cells. Transplanted memory cells respond to CGG (b). Thirty-seven days after the transplant, recipients and unmanipulated ATX BALB/c mice were immunized with CGG or PCC in CFA. Two weeks later, draining LN cells were collected, depleted of residual recipient cells, and rechallenged with 50 μg CGG in vitro in a standard proliferation assay. Cells were pooled from all animals (n = 3–7) of an experimental group: untransplanted ATX control, BM alone, BM plus unfractionated CD4 cells, BM plus CD25–CD4+ memory cells. Background counts (no antigen) were subtracted from plotted data. P = 0.0002 for proliferation to CGG for BM plus memory cells versus BM plus unfractionated CD4 cells. P < 0.0001 for BM plus memory cells versus BM alone. Error bars indicate standard deviation of samples run in triplicate.
Comment in
- Pleasant memories: remembering immune protection while forgetting about graft-versus-host disease.
Sondel PM, Buhtoiarov IN, DeSantes K. Sondel PM, et al. J Clin Invest. 2003 Jul;112(1):25-7. doi: 10.1172/JCI19095. J Clin Invest. 2003. PMID: 12840055 Free PMC article.
Similar articles
- Post-hematopoietic cell transplantation control of graft-versus-host disease by donor CD425 T cells to allow an effective graft-versus-leukemia response.
Jones SC, Murphy GF, Korngold R. Jones SC, et al. Biol Blood Marrow Transplant. 2003 Apr;9(4):243-56. doi: 10.1053/bbmt.2003.50027. Biol Blood Marrow Transplant. 2003. PMID: 12720217 - Pleasant memories: remembering immune protection while forgetting about graft-versus-host disease.
Sondel PM, Buhtoiarov IN, DeSantes K. Sondel PM, et al. J Clin Invest. 2003 Jul;112(1):25-7. doi: 10.1172/JCI19095. J Clin Invest. 2003. PMID: 12840055 Free PMC article. - Host-reactive CD8+ memory stem cells in graft-versus-host disease.
Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. Zhang Y, et al. Nat Med. 2005 Dec;11(12):1299-305. doi: 10.1038/nm1326. Epub 2005 Nov 20. Nat Med. 2005. PMID: 16288282 - Ex vivo selection of recipient-type alloantigen-specific CD4(+)CD25(+) immunoregulatory T cells for the control of graft-versus-host disease after allogeneic hematopoietic stem-cell transplantation.
Trenado A, Fisson S, Braunberger E, Klatzmann D, Salomon BL, Cohen JL. Trenado A, et al. Transplantation. 2004 Jan 15;77(1 Suppl):S32-4. doi: 10.1097/01.TP.0000106470.07410.CA. Transplantation. 2004. PMID: 14726768 Review.
Cited by
- Impact of Pre-Transplant Anti-T Cell Globulin (ATG) on Immune Recovery after Myeloablative Allogeneic Peripheral Blood Stem Cell Transplantation.
Servais S, Menten-Dedoyart C, Beguin Y, Seidel L, Gothot A, Daulne C, Willems E, Delens L, Humblet-Baron S, Hannon M, Baron F. Servais S, et al. PLoS One. 2015 Jun 22;10(6):e0130026. doi: 10.1371/journal.pone.0130026. eCollection 2015. PLoS One. 2015. PMID: 26098781 Free PMC article. - Low-dose anti-thymocyte globulin plus low-dose posttransplant cyclophosphamide as graft-versus-host disease prophylaxis in haploidentical peripheral blood stem cell transplantation combined with unrelated cord blood for patients with hematologic malignancies: a prospective, phase II study.
Yang J, Jiang J, Cai Y, Li S, Wan L, Zhu J, Liu H, Shao S, Bai H, Wang C, Song X. Yang J, et al. Bone Marrow Transplant. 2019 Jul;54(7):1049-1057. doi: 10.1038/s41409-018-0382-3. Epub 2018 Nov 16. Bone Marrow Transplant. 2019. PMID: 30446741 Free PMC article. Clinical Trial. - Immunological priming of mesenchymal stromal/stem cells and their extracellular vesicles augments their therapeutic benefits in experimental graft-versus-host disease via engagement of PD-1 ligands.
Hackel A, Vollmer S, Bruderek K, Lang S, Brandau S. Hackel A, et al. Front Immunol. 2023 Feb 16;14:1078551. doi: 10.3389/fimmu.2023.1078551. eCollection 2023. Front Immunol. 2023. PMID: 36875112 Free PMC article. - Reconstructing immunity after allogeneic transplantation.
Giver CR, Li JM, Hossain MS, Lonial S, Waller EK. Giver CR, et al. Immunol Res. 2004;29(1-3):269-82. doi: 10.1385/IR:29:1-3:269. Immunol Res. 2004. PMID: 15181288 Review. - TCF-1 Is Required for CD4 T Cell Persistence Functions during AlloImmunity.
Mammadli M, Suo L, Sen JM, Karimi M. Mammadli M, et al. Int J Mol Sci. 2023 Feb 21;24(5):4326. doi: 10.3390/ijms24054326. Int J Mol Sci. 2023. PMID: 36901757 Free PMC article.
References
- Mackall CL, Gress RE. Pathways of T-cell regeneration in mice and humans: implications for bone marrow transplantation and immunotherapy. Immunol. Rev. 1997;157:61–72. - PubMed
- Jameson SC. Maintaining the norm: T-cell homeostasis. Nat. Rev. Immunol. 2002;2:547–556. - PubMed
- Walters MC, et al. Bone marrow transplantation for sickle cell disease. N. Engl. J. Med. 1996;335:369–376. - PubMed
- Lucarelli G, et al. Bone marrow transplantation in adult thalassemic patients. Blood. 1999;93:1164–1167. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL066279/HL/NHLBI NIH HHS/United States
- K08 HL03979-02/HL/NHLBI NIH HHS/United States
- R01 HL66279/HL/NHLBI NIH HHS/United States
- T32 AI07109-23-25/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous