Assessment by meta-analysis of PCR for diagnosis of smear-negative pulmonary tuberculosis - PubMed (original) (raw)
Meta-Analysis
Assessment by meta-analysis of PCR for diagnosis of smear-negative pulmonary tuberculosis
Olga L Sarmiento et al. J Clin Microbiol. 2003 Jul.
Abstract
We conducted a meta-analysis to assess the performance of PCR for the diagnosis of smear-negative pulmonary tuberculosis (SPT) and to identify factors that account for differences in the diagnostic accuracy of different studies. Studies published before February 2002 were included if sensitivity and specificity of PCR in smear-negative respiratory or gastric-aspirate specimens could be calculated. Analysis was conducted by using summary receiver operating characteristics models. Sensitivity and specificity ranged from 9 to 100% and from 25 to 100%, respectively. Fewer than 40% of the 50 studies reported results by number of patients, reported clinical characteristics of patients, or used as a reference standard combined culture and clinical criteria. Studies that included bronchial specimens showed higher accuracy than studies that evaluated only sputum specimens or included gastric aspirates. Studies that did not report that tests were applied blindly showed higher accuracy than those reporting blind testing. Increased sensitivity due to the use of DNA purification methods was associated with decreased specificity. Studies published after 1995, using Amplicor or dUTP-UNG, were associated with an increase in specificity at the expense of lower sensitivity. We concluded that PCR is not consistently accurate enough to be routinely recommended for the diagnosis of SPT. However, PCR of bronchial specimens could be useful in highly suspicious SPT cases. Studies not reporting blind testing are likely to overestimate accuracy of PCR. Future evaluation of PCR accuracy should be conducted by patient and type of respiratory specimen, blindly, by using a reference standard that combines culture and clinical criteria and addresses the issue of how patient characteristics affect PCR accuracy.
Figures
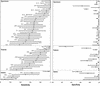
FIG. 1.
Point estimates and 95% confidence intervals of sensitivity and specificity of PCR for detection of M. tuberculosis in smear-negative respiratory and gastric specimens. Specimen studies included studies in which respiratory specimens (sputum, tracheal aspirates, bronchial washings, and bronchoalveolar lavage) were the unit of analysis. Patient studies included studies in which the patients, whose respiratory specimens were evaluated, were the unit of analysis. Gastric studies included studies in which gastric aspirates or respiratory specimens plus gastric aspirates were evaluated. The number of studies totals 51 because 1 study reported results by both gastric aspirates and respiratory specimens. The numbers in parentheses correspond to study references.
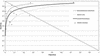
FIG. 2.
Plot in the ROC space of accuracy estimates for PCR for the detection of M. tuberculosis in smear-negative respiratory specimens. Each of the 16 studies that were analyzed by patients is indicated by a triangle. ROC curves are shown for studies that analyzed bronchial specimens or tracheal specimens (thin line), for studies that analyzed only sputum specimens (broken line), and for all 16 studies (thick line). The intersection of the diagonal line from the upper left corner to the lower right corner of the ROC space and the SROC curve corresponds to the maximum joint sensitivity and specificity value.
Similar articles
- Performance of microbiological tests for tuberculosis diagnostic according to the type of respiratory specimen: A 10-year retrospective study.
Boldi MO, Denis-Lessard J, Neziri R, Brouillet R, von-Garnier C, Chavez V, Mazza-Stalder J, Jaton K, Greub G, Opota O. Boldi MO, et al. Front Cell Infect Microbiol. 2023 Mar 2;13:1131241. doi: 10.3389/fcimb.2023.1131241. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 36936773 Free PMC article. - Comparison of amplicor and 32-kilodalton PCR for detection of Mycobacterium tuberculosis from sputum specimens.
Soini H, Agha SA, El-Fiky A, Viljanen MK. Soini H, et al. J Clin Microbiol. 1996 Jul;34(7):1829-30. doi: 10.1128/JCM.34.7.1829-1830.1996. J Clin Microbiol. 1996. PMID: 8784603 Free PMC article. - Diagnostic value of the Amplicor PCR assay for initial diagnosis and assessment of treatment response for pulmonary tuberculosis.
Iinuma Y, Ichiyama S, Yamori S, Oohama J, Takagi N, Hasegawa Y, Shimokata K, Nakashima N. Iinuma Y, et al. Microbiol Immunol. 1998;42(4):281-7. doi: 10.1111/j.1348-0421.1998.tb02284.x. Microbiol Immunol. 1998. PMID: 9623915 - Clinical evaluation of the Roche AMPLICOR PCR Mycobacterium tuberculosis test for detection of M. tuberculosis in respiratory specimens.
Bergmann JS, Woods GL. Bergmann JS, et al. J Clin Microbiol. 1996 May;34(5):1083-5. doi: 10.1128/jcm.34.5.1083-1085.1996. J Clin Microbiol. 1996. PMID: 8727880 Free PMC article. - An Evaluation of The Diagnostic Value of Sputum Smears Microscopy and Pcr Relative to Sputum Culture in The Diagnosis of Pulmonary Tuberculosis: A Systematic Review and Meta-Analysis in Iran.
Rahmati S, Bahrampour A, Nasehi M, Mirzazadeh A, Ghaderi H, Shahesmaeili A. Rahmati S, et al. Med J Islam Repub Iran. 2022 Sep 27;36:112. doi: 10.47176/mjiri.36.112. eCollection 2022. Med J Islam Repub Iran. 2022. PMID: 36447544 Free PMC article. Review.
Cited by
- Use of amplified Mycobacterium tuberculosis direct test (Gen-probe Inc., San Diego, CA, USA) in the diagnosis of tubercular synovitis and early arthritis of knee joint.
Aggarwal VK, Nair D, Khanna G, Verma J, Sharma VK, Batra S. Aggarwal VK, et al. Indian J Orthop. 2012 Sep;46(5):531-5. doi: 10.4103/0019-5413.101039. Indian J Orthop. 2012. PMID: 23162145 Free PMC article. - Using giant African pouched rats to detect tuberculosis in human sputum samples: 2009 findings.
Poling A, Weetjens BJ, Cox C, Mgode G, Jubitana M, Kazwala R, Mfinanga GS, Huis In 't Veld D. Poling A, et al. Am J Trop Med Hyg. 2010 Dec;83(6):1308-10. doi: 10.4269/ajtmh.2010.10-0180. Am J Trop Med Hyg. 2010. PMID: 21118940 Free PMC article. - Evaluation of a real-time PCR assay performance to detect Mycobacterium tuberculosis, rifampicin, and isoniazid resistance in sputum specimens: a multicenter study in two major cities of Indonesia.
Parwati I, Chaidir L, Yunus M, Montain MM, Budhiarko D, Selasih SF, Ristandi RB, Rachman RW, Nurhayati RD, Pambudi I, Budiyati AD. Parwati I, et al. Front Microbiol. 2024 May 10;15:1372647. doi: 10.3389/fmicb.2024.1372647. eCollection 2024. Front Microbiol. 2024. PMID: 38800757 Free PMC article. - Ocular tuberculosis: Where are we today?
Testi I, Agrawal R, Mehta S, Basu S, Nguyen Q, Pavesio C, Gupta V. Testi I, et al. Indian J Ophthalmol. 2020 Sep;68(9):1808-1817. doi: 10.4103/ijo.IJO_1451_20. Indian J Ophthalmol. 2020. PMID: 32823397 Free PMC article. Review. - Cost-effectiveness analysis of PCR for the rapid diagnosis of pulmonary tuberculosis.
Scherer LC, Sperhacke RD, Ruffino-Netto A, Rossetti ML, Vater C, Klatser P, Kritski AL. Scherer LC, et al. BMC Infect Dis. 2009 Dec 31;9:216. doi: 10.1186/1471-2334-9-216. BMC Infect Dis. 2009. PMID: 20043842 Free PMC article.
References
- Almeda, J., A. Garcia, J. Gonzalez, L. Quinto, P. J. Ventura, R. Vidal, G. Rufi, J. A. Martinez, M. T. Jimenez de Anta, A. Trilla, and P. L. Alonso. 2000. Clinical evaluation of an in-house IS6110 polymerase chain reaction for diagnosis of tuberculosis. Eur. J. Clin. Microbiol. Infect. Dis. 19:859-867. - PubMed
- Al Zahrani, K., H. Al Jahdali, L. Poirier, P. Rene, and D. Menzies. 2001. Yield of smear, culture and amplification tests from repeated sputum induction for the diagnosis of pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 5:855-860. - PubMed
- American Thoracic Society Workshop. 1997. Rapid diagnostic tests for tuberculosis: what is the appropriate use? Am. J. Respir. Crit. Care Med. 155:1804-1814. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources