Postsynaptic density-95 mimics and occludes hippocampal long-term potentiation and enhances long-term depression - PubMed (original) (raw)
Postsynaptic density-95 mimics and occludes hippocampal long-term potentiation and enhances long-term depression
Valentin Stein et al. J Neurosci. 2003.
Abstract
Previous studies have shown that overexpression of the protein PSD-95 (postsynaptic density-95) selectively enhances AMPA receptor-mediated synaptic responses in hippocampal pyramidal cells. To determine whether this effect is related to synaptic plasticity at these synapses, we examined whether PSD-95 expression mimics long-term potentiation (LTP), and also whether it influences LTP and long-term depression (LTD) in hippocampal slice cultures. Using simultaneous recording from transfected or infected cells and control pyramidal cells, we found that PSD-95, similar to LTP, increases the amplitude and frequency of miniature EPSCs. It also converts silent synapses to functional synapses, as does LTP. In addition, LTP is completely occluded in cells expressing PSD-95, whereas LTD is greatly enhanced. These results suggest that common mechanisms are involved in controlling synaptic AMPA receptors by PSD-95 and synaptic plasticity.
Figures
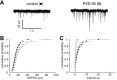
Figure 1.
Expression of PSD-95 enhances the amplitude and frequency of mEPSCs. A, Five superimposed sample traces showing mEPSCs from a control cell (left) and a PSD-95-expressing cell (right). Note that there are many more events in the PSD-95-expressing cell, and that some of the events are larger than events recorded in a control cell. B, Cumulative frequency distributions of the amplitudes of mEPSCs recorded from control cells (closed circles) and PSD-95-expressing cells (open circles) (n = 7). C, Cumulative frequency distributions of the interevent intervals of mEPSCs recorded in control cells (closed circles) and PSD-95-expressing cells (open circles).
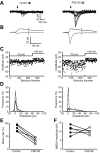
Figure 2.
Expression of PSD-95 inserts AMPA receptors into silent synapses. A, Ten superimposed traces showing responses to minimal stimulation from a control cell (left) and a PSD-95-expressing cell (right). Arrows indicate when the stimulus was delivered. Note that there are many fewer failures, and that the responses are much larger in the PSD-95-expressing cell. B, Averaged records of all traces at –70 and +40 mV. Note that the NMDA component is the same in the two cells, whereas the AMPA component is much larger in the PSD-95-expressing cell. The NMDA component was measured 55 msec after the stimulus, as indicated by the dashed line. C, Plots of EPSC amplitude versus trial number at –70 and +40 mV for the control cell (left) and PSD-95-expressing cell (right). D, EPSC amplitude distribution (solid line) and noise (broken line) in the control cell (left) and the PSD-95 expressing cell (right). See Materials and Methods for calculating noise. E, A pair-wise comparison of the failure rate between control and PSD-95-expressing cells. See Materials and Methods for estimating failure rate. F, A pair-wise comparison of the size of the NMDA EPSC recorded at +40 mV. The symbol for each pair is the same as that used in E.
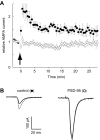
Figure 3.
Expression of PSD-95 occludes LTP. A, Graph comparing LTP recorded simultaneously from control neurons (closed circles) and neurons expressing PSD-95 (open circles). The number of cells for both graphs is initially 10 and decreases to five by the end. Arrow indicates the time of pairing. B, Sample records from a pair of neurons showing EPSCs recorded before pairing (solid lines) and after pairing (dotted lines).
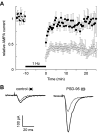
Figure 4.
Expression of PSD-95 enhances LTD. A, Graph comparing LTD recorded simultaneously from control neurons (closed circles) and from neurons expressing PSD-95 (open circles). The number of cells for the control graph is initially 11 and decreases to six by the end of the experiments. For the graph of the cells expressing PSD-95, the number of cells is initially 11 and decreases to five by the end of the experiment. B, Sample records from a pair of neurons showing EPSCs recorded before pairing (solid lines) and after pairing (dotted lines).
Similar articles
- Postsynaptic density 95 controls AMPA receptor incorporation during long-term potentiation and experience-driven synaptic plasticity.
Ehrlich I, Malinow R. Ehrlich I, et al. J Neurosci. 2004 Jan 28;24(4):916-27. doi: 10.1523/JNEUROSCI.4733-03.2004. J Neurosci. 2004. PMID: 14749436 Free PMC article. - Opposing effects of PSD-93 and PSD-95 on long-term potentiation and spike timing-dependent plasticity.
Carlisle HJ, Fink AE, Grant SG, O'Dell TJ. Carlisle HJ, et al. J Physiol. 2008 Dec 15;586(24):5885-900. doi: 10.1113/jphysiol.2008.163469. Epub 2008 Oct 20. J Physiol. 2008. PMID: 18936077 Free PMC article. - Postsynaptic expression of a new calcium pathway in hippocampal CA3 neurons and its influence on mossy fiber long-term potentiation.
Kakegawa W, Yamada N, Iino M, Kameyama K, Umeda T, Tsuzuki K, Ozawa S. Kakegawa W, et al. J Neurosci. 2002 Jun 1;22(11):4312-20. doi: 10.1523/JNEUROSCI.22-11-04312.2002. J Neurosci. 2002. PMID: 12040036 Free PMC article. - AMPA receptor regulation during synaptic plasticity in hippocampus and neocortex.
Lee HK, Kirkwood A. Lee HK, et al. Semin Cell Dev Biol. 2011 Jul;22(5):514-20. doi: 10.1016/j.semcdb.2011.06.007. Epub 2011 Aug 12. Semin Cell Dev Biol. 2011. PMID: 21856433 Free PMC article. Review. - Postsynaptic depolarization requirements for LTP and LTD: a critique of spike timing-dependent plasticity.
Lisman J, Spruston N. Lisman J, et al. Nat Neurosci. 2005 Jul;8(7):839-41. doi: 10.1038/nn0705-839. Nat Neurosci. 2005. PMID: 16136666 Review.
Cited by
- Acute inactivation of PSD-95 destabilizes AMPA receptors at hippocampal synapses.
Yudowski GA, Olsen O, Adesnik H, Marek KW, Bredt DS. Yudowski GA, et al. PLoS One. 2013;8(1):e53965. doi: 10.1371/journal.pone.0053965. Epub 2013 Jan 16. PLoS One. 2013. PMID: 23342049 Free PMC article. - Neddylation inhibition impairs spine development, destabilizes synapses and deteriorates cognition.
Vogl AM, Brockmann MM, Giusti SA, Maccarrone G, Vercelli CA, Bauder CA, Richter JS, Roselli F, Hafner AS, Dedic N, Wotjak CT, Vogt-Weisenhorn DM, Choquet D, Turck CW, Stein V, Deussing JM, Refojo D. Vogl AM, et al. Nat Neurosci. 2015 Feb;18(2):239-51. doi: 10.1038/nn.3912. Epub 2015 Jan 12. Nat Neurosci. 2015. PMID: 25581363 - The cell adhesion protein dystroglycan affects the structural remodeling of dendritic spines.
Figiel I, Bączyńska E, Wójtowicz T, Magnowska M, Buszka A, Bijata M, Włodarczyk J. Figiel I, et al. Sci Rep. 2022 Feb 15;12(1):2506. doi: 10.1038/s41598-022-06462-7. Sci Rep. 2022. PMID: 35169214 Free PMC article. - SynGAP regulates synaptic strength and mitogen-activated protein kinases in cultured neurons.
Rumbaugh G, Adams JP, Kim JH, Huganir RL. Rumbaugh G, et al. Proc Natl Acad Sci U S A. 2006 Mar 21;103(12):4344-51. doi: 10.1073/pnas.0600084103. Epub 2006 Mar 14. Proc Natl Acad Sci U S A. 2006. PMID: 16537406 Free PMC article. - Altered aquaporin 4 expression in muscles of Fukuyama-type congenital muscular dystrophy.
Wakayama Y, Jimi T, Inoue M, Kojima H, Yamashita S, Kumagai T, Murahashi M, Hara H, Shibuya S. Wakayama Y, et al. Virchows Arch. 2003 Dec;443(6):761-7. doi: 10.1007/s00428-003-0887-y. Epub 2003 Aug 26. Virchows Arch. 2003. PMID: 12942324
References
- Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ, Bredt DS, Nicoll RA ( 2000) Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature 408: 936–943. - PubMed
- DiCiommo DP, Bremner R ( 1998) Rapid, high level protein production using DNA-based Semliki Forest virus vectors. J Biol Chem 273: 18060–18066. - PubMed
- Ehrlich ID, Malinow R ( 2002) PSD-95 mimics, occludes and dominant negative forms block LTP in hippocampal slice cultures. Soc Neurosci Abstr 28: 713.2.
- El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS ( 2000) PSD-95 involvement in maturation of excitatory synapses. Science 290: 1364 –1368. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources