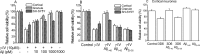The production of amyloid beta peptide is a critical requirement for the viability of central neurons - PubMed (original) (raw)
The production of amyloid beta peptide is a critical requirement for the viability of central neurons
Leigh D Plant et al. J Neurosci. 2003.
Abstract
The amyloid beta peptide (Abeta) is a product of the sequential gamma- and beta-secretase cleavage of amyloid precursor protein. Inhibitors of secretase enzymes have been proposed as a potential therapeutic strategy in the treatment of Alzheimer's disease. Here, we investigate the effect of inhibiting these key enzymes on the viability of a range of cell types. Treatment of rat cortical neurons for 24 hr with secretase inhibitors or an antibody that binds Abeta resulted in a marked reduction in cell viability, as measured by MTT reduction. Incubation with secretase inhibitors caused similar effects on other neuronal cell types (rat cerebellar granule neurons and the human SH-SY5Y cell line). Interestingly, rat astrocytes and a number of non-neuronal cell lines investigated (HEK293, DDT1-FM2, and human teratorhabdoid tumor cells) were unaffected by incubation with secretase inhibitors. The coincubation of Abeta1-40 prevented the toxicity of secretase inhibitors in neuronal cells. Abeta1-40 was protective in a concentration-dependent manner, and its effects were significant at concentrations as low at 10 pm. Importantly, the protective effects of Abeta were Abeta size-form specific, with the Abeta1-42 size form affording limited protection and the Abeta25-35 size form having very little protective effect. The present study demonstrates that inhibition of beta-or gamma-secretase activity induces death in neuronal cells. Importantly, this toxicity, which our data suggest is a consequence of a decline in neuronal Abeta levels, was absent in non-neuronal cells. This study further supports a key physiological role for the enigmatic Abeta peptide.
Figures
Figure 1.
Effect of γ-secretase inhibition on cell morphology. Phase-contrast photomicrographs of cortical neurons after 24 hr incubation with 1 n
m
Aβ1–40 (A), 24 hr incubation with 10 μ
m
γ-IV (B), and 24 hr coincubation of 1 n
m
Aβ1–40 and 10 μ
m
γ-IV (C). Scale bar, 100 μm.
Figure 2.
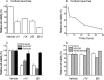
Effect of secretase inhibition on cell viability. A, Incubation for 24 hr with γ- or β-secretase inhibitors on the viability of cortical neurons (n = 9 for each condition). B, Time dependence of γ-IV toxicity on cortical cultures compared with vehicle-treated controls. Values are the mean of four experiments for each time point. C, Effect of 24 hr incubation with secretase inhibitors on cell death in cerebellar granule neurons (open bars; n = 9), the SH-SY5Y cell line (hatched bars; _n_=9), and rat astrocytes cultures (filled bars; n = 9). D, Effect of 48 hr incubation with secretase inhibitors on the viability of non-neuronal cell lines DDT1-FM2 (open bars; n = 4), HEK293 (hatched bars; n = 4), and human teratorhabdoid tumor cells (filled bars; _n_=4). γ-IX, γ-secretase inhibitor.
Figure 3.
Effect of secretase inhibition on Aβ levels in neurons. Fluorescence photomicrographs of cerebellar granule neurons under control conditions (A), after 24 hr incubation with 10 μ
m
γ-IV (B), and after 24 hr incubation with 100 n
m
βSI (C). Scale bar, 30 μm.
Figure 4.
Effect of amyloid proteins on γ-secretase inhibitor neurotoxicity. A, Effect of 24 hr incubation with γ-secretase inhibitor on the viability of cortical neurons (open bars; n = 9), cerebellar granule neurons (striped bars; n = 9), and the SH-SY5Y cell line (hatched bars; n = 9) in the presence of different concentrations of Aβ1–40. B, Effect of 24 hr incubation with γ-secretase inhibitor (10 μ
m
) on the viability of cortical neurons (open bars; n = 9), cerebellar granule neurons (striped bars; n = 9), and the SH-SY5Y cell line (hatched bars; n = 9) in the presence of different Aβ size forms at a concentration of 1 n
m
in each case. C, Toxicity of the monoclonal antibody 3D6 (1 μg/ml, 24 hr) on cortical neurons. Toxicity was reversed by coincubation with Aβ1–40 (1 n
m
) but not Aβ25–35 (1 n
m
). Aβ peptides alone had no effect on cell survival. In each case, n = 4.
Similar articles
- A distinct ER/IC gamma-secretase competes with the proteasome for cleavage of APP.
Skovronsky DM, Pijak DS, Doms RW, Lee VM. Skovronsky DM, et al. Biochemistry. 2000 Feb 1;39(4):810-7. doi: 10.1021/bi991728z. Biochemistry. 2000. PMID: 10651647 - Intraneuronal amyloid-beta1-42 production triggered by sustained increase of cytosolic calcium concentration induces neuronal death.
Pierrot N, Ghisdal P, Caumont AS, Octave JN. Pierrot N, et al. J Neurochem. 2004 Mar;88(5):1140-50. doi: 10.1046/j.1471-4159.2003.02227.x. J Neurochem. 2004. PMID: 15009669 - Selenium and Zinc against Aβ25-35-Induced Cytotoxicity and Tau Phosphorylation in PC12 Cells and Inhibits γ-cleavage of APP.
Li GZ, Liu F, Xu C, Li JY, Xu YJ. Li GZ, et al. Biol Trace Elem Res. 2018 Aug;184(2):442-449. doi: 10.1007/s12011-017-1162-4. Epub 2017 Oct 28. Biol Trace Elem Res. 2018. PMID: 29081063 - Proton myo-inositol cotransporter is a novel γ-secretase associated protein that regulates Aβ production without affecting Notch cleavage.
Teranishi Y, Inoue M, Yamamoto NG, Kihara T, Wiehager B, Ishikawa T, Winblad B, Schedin-Weiss S, Frykman S, Tjernberg LO. Teranishi Y, et al. FEBS J. 2015 Sep;282(17):3438-51. doi: 10.1111/febs.13353. Epub 2015 Jul 14. FEBS J. 2015. PMID: 26094765 Review. - BACE1: the beta-secretase enzyme in Alzheimer's disease.
Vassar R. Vassar R. J Mol Neurosci. 2004;23(1-2):105-14. doi: 10.1385/JMN:23:1-2:105. J Mol Neurosci. 2004. PMID: 15126696 Review.
Cited by
- Neuronal activity and secreted amyloid β lead to altered amyloid β precursor protein and presenilin 1 interactions.
Li X, Uemura K, Hashimoto T, Nasser-Ghodsi N, Arimon M, Lill CM, Palazzolo I, Krainc D, Hyman BT, Berezovska O. Li X, et al. Neurobiol Dis. 2013 Feb;50:127-34. doi: 10.1016/j.nbd.2012.10.002. Epub 2012 Oct 12. Neurobiol Dis. 2013. PMID: 23064434 Free PMC article. - Staying connected: synapses in Alzheimer disease.
Lee HG, Moreira PI, Zhu X, Smith MA, Perry G. Lee HG, et al. Am J Pathol. 2004 Nov;165(5):1461-4. doi: 10.1016/S0002-9440(10)63404-9. Am J Pathol. 2004. PMID: 15509517 Free PMC article. Review. No abstract available. - Neurogenesis Is Increased in Human Neural Stem Cells by Aβ40 Peptide.
Bernabeu-Zornoza A, Coronel R, Palmer C, Martín A, López-Alonso V, Liste I. Bernabeu-Zornoza A, et al. Int J Mol Sci. 2022 May 22;23(10):5820. doi: 10.3390/ijms23105820. Int J Mol Sci. 2022. PMID: 35628629 Free PMC article. - Amyloid-beta 1-40 is associated with alterations in NG2+ pericyte population ex vivo and in vitro.
Schultz N, Brännström K, Byman E, Moussaud S, Nielsen HM; Netherlands Brain Bank; Olofsson A, Wennström M. Schultz N, et al. Aging Cell. 2018 Jun;17(3):e12728. doi: 10.1111/acel.12728. Epub 2018 Feb 17. Aging Cell. 2018. PMID: 29453790 Free PMC article. - The keystone of Alzheimer pathogenesis might be sought in Aβ physiology.
Puzzo D, Gulisano W, Arancio O, Palmeri A. Puzzo D, et al. Neuroscience. 2015 Oct 29;307:26-36. doi: 10.1016/j.neuroscience.2015.08.039. Epub 2015 Aug 24. Neuroscience. 2015. PMID: 26314631 Free PMC article. Review.
References
- Abbenante G, Kovacs DM, Leung DL, Craik DJ, Tanzi RE, Fairlie DP ( 2000) Inhibitors of beta-amyloid formation based on the beta-secretase cleavage site. Biochem Biophys Res Commun 268: 133–135. - PubMed
- Annaert WG, Esselens C, Baert V, Boeve C, Snellings G, Cupers P, Craessaerts K and De Strooper B ( 2001) Interaction with telencephalin and the amyloid precursor protein predicts a ring structure for presenilins. Neuron 32: 579–589. - PubMed
- Beher D, Wrigley JD, Nadin A, Evin G, Masters CL, Harrison T, Castro JL, Shearman MS ( 2001) Pharmacological knock-down of the presenilin 1 heterodimer by a novel gamma-secretase inhibitor: implications for presenilin biology. J Biol Chem 276: 45394–45402. - PubMed
- Cai H, Wang Y, McCarthy D, Wen H, Borchelt DR, Price DL, Wong PC ( 2001) BACE1 is the major beta-secretase for generation of Aβ peptides by neurons. Nat Neurosci 4: 233–234. - PubMed
- Cummings BJ, Cotman CW ( 1995) Image analysis of beta-amyloid load in Alzheimer's disease and relation to dementia severity. Lancet 346: 1524–1528. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources