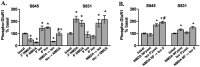NMDA and beta1-adrenergic receptors differentially signal phosphorylation of glutamate receptor type 1 in area CA1 of hippocampus - PubMed (original) (raw)
NMDA and beta1-adrenergic receptors differentially signal phosphorylation of glutamate receptor type 1 in area CA1 of hippocampus
Amanda M Vanhoose et al. J Neurosci. 2003.
Abstract
Glutamatergic synaptic transmission is mediated primarily through the AMPA-type glutamate receptor (AMPAR); the regulation of this receptor underlies many forms of synaptic plasticity. In particular, phosphorylation of GluR1, an AMPAR subunit, by PKA at serine 845 (S845) increases peak open channel probability and is permissive for both the synaptic expression of the receptor and NMDA-receptor (NMDAR)-dependent long-term potentiation (LTP). Robust NMDAR activation activates PKA as well as other signaling enzymes; however, we find that maximal NMDAR activation dephosphorylates GluR1 at the PKA site S845. Coincident inhibition of phosphatases blocks NMDAR-induced dephosphorylation of S845, but surprisingly does not promote PKA phosphorylation at this site. However, we find that phosphorylation of S845 is increased by the activation of a Gs-coupled receptor, the beta1-adrenergic receptor. Interestingly, this divergent signaling occurs despite a more robust coupling of the NMDAR to cAMP generation. In addition, NMDAR activation plays a dominant role in S845 regulation, because activation of beta1AR after NMDAR activation has no detectable effect on S845 phosphorylation. These data (1) demonstrate highly specific coupling between these receptors and this substrate, (2) provide an example of a substrate critical in NMDAR-dependent LTP that is incompletely regulated by the NMDAR, and (3) highlight the importance of identifying the physiological signals that regulate these critical synaptic substrates.
Figures
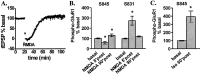
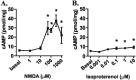
Figure 1.
NMDAR activation decreases the S845 and increases the S831 phosphorylation of GluR1 in area CA1 from mouse hippocampal slices. A, Differences from basal levels in GluR1 phosphorylation at S845 and S831 after pretreatment with rolipram (ro; 0.3μ
m
, 30 min) or vehicle (0.01% DMSO) followed by treatment with NMDA (300μ
m
, 3 min) (n = 4–5). B, NMDA (300μ
m
, 3 min) increases cAMP levels in the presence of rolipram (0.3 μ
m
, 30 min pretreatment) (n = 5). C, Differences from basal levels in GluR1 phosphorylation at S845 and S831 after treatment with varying doses of NMDA (10–300 μ
m
, 3 min) (n = 7–14). D, Differences from basal levels in GluR1 phosphorylation at S845and S831 after pretreatment with tetrodotoxin (TTX;1μ
m
,30 min) followed by treatment with NMDA (300μ
m
, 3 min) (n = 4–7). *p < 0.05 compared with basal levels; #p < 0.05 compared with rolipram or TTX.
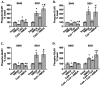
Figure 3.
NMDAR activation induces a transient change in synaptic transmission and GluR1 phosphorylation, whereas βAR activation induces a long-lasting change in GluR1 phosphorylation.A,fEPSP recordings measured in area CA1 after treatment with NMDA (300 μ
m
, 3 min) (n = 5). Differences from basal in GluR1 phosphorylation at S845 and S831 at 5 min and 90 min after treatment with NMDA (300 μ
m
, 3 min) in an interface perfusion chamber (B) (n = 7–19) and at 60 min after treatment with isoproterenol in an interface perfusion chamber (C) (1 μ
m
, 15 min) (n = 11). *p < 0.05 compared with basal levels.
Figure 6.
NMDAR is dominant overβAR in the regulation of GluR1 phosphorylation in area CA1 from mouse hippocampal slices. A, Differences from basal levels in GluR1 phosphorylation at S845 and S831 after treatment with NMDA (300 μ
m
, 3 and 6 min) or isoproterenol (Iso; 1 μ
m
, 3 and 6 min) in submerged chambers. NMDA → isoproterenol indicates that NMDA was added initially (6 min) and isoproterenol was coapplied with NMDA during the final 3 min. Isoproterenol → NMDA indicates the opposite order of drug application (n = 4–6). *p < 0.05 compared with basal levels; #p < 0.05 compared with NMDA 3′; +p < 0.05 compared with isoproterenol 6′. B, Differences from basal levels in GluR1 phosphorylation at S845 and S831 after treatment with NMDA (300μ
m
, 3 min) and isoproterenol (1μ
m
, 3 min) in an interface perfusion chamber at 5 and 90 min after drug application. NMDA 90′ → isoproterenol 5′ indicates that NMDA was added initially, and 90 min later isoproterenol was added (n = 6). *p < 0.05 compared with basal levels; #p < 0.05 compared with NMDA 90′.
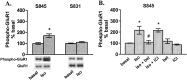
Figure 2.
β1AR activation increases S845 phosphorylation of GluR1 in area CA1 from mouse hippocampal slices. A, Differences from basal levels in GluR1 phosphorylation at S845 and S831 after treatment with isoproterenol (iso; 1 μ
m
, 3 min) (n = 3). B, Differences from basal levels in GluR1 phosphorylation at S845 and S831 after pretreatment with betaxolol (bet; 10 μ
m
, 10 min) or ICI-118,551(ICI;10μ
m
,10 min) followed by isoproterenol(100 n
m
,6 min)(_n_=4).*p<0.05 compared with basal levels; #p< 0.05 compared with isoproterenol.
Figure 4.
NMDAR and βAR activation dose-dependently increase cAMP levels in area CA1 from mouse hippocampal slices, but to differing magnitudes. A, Dose–response curve of NMDA (1 μ
m
to 1 m
m
, 3 min) induced changes in cAMP concentration relative to basal levels (n = 3–6). B, Dose–response curve of isoproterenol (1 n
m
to 10μ
m
, 3 min) induced changes in cAMP concentration relative to basal levels (n = 3–4). *p < 0.05 compared with basal levels.
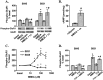
Figure 5.
Multiple phosphatases regulate NMDAR-stimulated changes in GluR1 phosphorylation in area CA1 from mouse hippocampal slices. A, Differences from basal levels in GluR1 phosphorylation at S845 and S831 after pretreatment with calyculin A (CalA; 1 μ
m
, 30 min) or vehicle (0.1% DMSO) followed by treatment with NMDA (300 μ
m
, 3 min) (n = 10–11). B, Differences from basal levels in GluR1 phosphorylation at S845 and S831 after pretreatment with cypermethrin (Cyp; 10μ
m
, 30 min) or vehicle (0.1% DMSO) followed by treatment with NMDA (300μ
m
, 3 min) (n = 4–5). C, Differences from basal levels in GluR1 phosphorylation at S845 and S831 after pretreatment with cyclosporinA (CsA;1μ
m
,30 min) or vehicle (0.1%DMSO) followed by treatment with NMDA (300 μ
m
, 3 min) (n = 7). D, Differences from basal levels in GluR1 phosphorylation at S845 and S831 after simultaneous pretreatment with calyculin A plus cyclosporin A (1 μ
m
each, 30 min) or vehicle (0.2% DMSO) followed by treatment with NMDA (300μ
m
, 3 min) (n = 4–5). *p < 0.05 compared with basal levels; #p < 0.05 compared with calyculin A, cypermethrin, cyclosporin A, and calyculin A plus cyclosporin A.
Similar articles
- Novel blockade of protein kinase A-mediated phosphorylation of AMPA receptors.
Vanhoose AM, Clements JM, Winder DG. Vanhoose AM, et al. J Neurosci. 2006 Jan 25;26(4):1138-45. doi: 10.1523/JNEUROSCI.3572-05.2006. J Neurosci. 2006. PMID: 16436600 Free PMC article. - Regulation of neuronal PKA signaling through AKAP targeting dynamics.
Dell'Acqua ML, Smith KE, Gorski JA, Horne EA, Gibson ES, Gomez LL. Dell'Acqua ML, et al. Eur J Cell Biol. 2006 Jul;85(7):627-33. doi: 10.1016/j.ejcb.2006.01.010. Epub 2006 Feb 28. Eur J Cell Biol. 2006. PMID: 16504338 Review. - NMDA receptor activation dephosphorylates AMPA receptor glutamate receptor 1 subunits at threonine 840.
Delgado JY, Coba M, Anderson CN, Thompson KR, Gray EE, Heusner CL, Martin KC, Grant SG, O'Dell TJ. Delgado JY, et al. J Neurosci. 2007 Nov 28;27(48):13210-21. doi: 10.1523/JNEUROSCI.3056-07.2007. J Neurosci. 2007. PMID: 18045915 Free PMC article. - 'Silent' priming of translation-dependent LTP by ß-adrenergic receptors involves phosphorylation and recruitment of AMPA receptors.
Tenorio G, Connor SA, Guévremont D, Abraham WC, Williams J, O'Dell TJ, Nguyen PV. Tenorio G, et al. Learn Mem. 2010 Nov 23;17(12):627-38. doi: 10.1101/lm.1974510. Print 2010 Dec. Learn Mem. 2010. PMID: 21097606 Free PMC article. - NMDA receptor-independent LTP in mammalian nervous system.
Alkadhi KA. Alkadhi KA. Prog Neurobiol. 2021 May;200:101986. doi: 10.1016/j.pneurobio.2020.101986. Epub 2021 Jan 2. Prog Neurobiol. 2021. PMID: 33400965 Review.
Cited by
- Control of βAR- and N-methyl-D-aspartate (NMDA) Receptor-Dependent cAMP Dynamics in Hippocampal Neurons.
Chay A, Zamparo I, Koschinski A, Zaccolo M, Blackwell KT. Chay A, et al. PLoS Comput Biol. 2016 Feb 22;12(2):e1004735. doi: 10.1371/journal.pcbi.1004735. eCollection 2016 Feb. PLoS Comput Biol. 2016. PMID: 26901880 Free PMC article. - β-adrenergic signaling broadly contributes to LTP induction.
Jȩdrzejewska-Szmek J, Luczak V, Abel T, Blackwell KT. Jȩdrzejewska-Szmek J, et al. PLoS Comput Biol. 2017 Jul 24;13(7):e1005657. doi: 10.1371/journal.pcbi.1005657. eCollection 2017 Jul. PLoS Comput Biol. 2017. PMID: 28742159 Free PMC article. - Assembly of a beta2-adrenergic receptor--GluR1 signalling complex for localized cAMP signalling.
Joiner ML, Lisé MF, Yuen EY, Kam AY, Zhang M, Hall DD, Malik ZA, Qian H, Chen Y, Ulrich JD, Burette AC, Weinberg RJ, Law PY, El-Husseini A, Yan Z, Hell JW. Joiner ML, et al. EMBO J. 2010 Jan 20;29(2):482-95. doi: 10.1038/emboj.2009.344. Epub 2009 Nov 26. EMBO J. 2010. PMID: 19942860 Free PMC article. - SAP97 controls the trafficking and resensitization of the beta-1-adrenergic receptor through its PDZ2 and I3 domains.
Nooh MM, Naren AP, Kim SJ, Xiang YK, Bahouth SW. Nooh MM, et al. PLoS One. 2013 May 16;8(5):e63379. doi: 10.1371/journal.pone.0063379. Print 2013. PLoS One. 2013. PMID: 23696820 Free PMC article. - Viagra for your synapses: Enhancement of hippocampal long-term potentiation by activation of beta-adrenergic receptors.
O'Dell TJ, Connor SA, Gelinas JN, Nguyen PV. O'Dell TJ, et al. Cell Signal. 2010 May;22(5):728-36. doi: 10.1016/j.cellsig.2009.12.004. Epub 2009 Dec 31. Cell Signal. 2010. PMID: 20043991 Free PMC article. Review.
References
- Bailey CH, Giustetto M, Huang YY, Hawkins RD, Kandel ER ( 2000) Is heterosynaptic modulation essential for stabilizing Hebbian plasticity and memory? Nat Rev Neurosci 1: 11-20. - PubMed
- Blitzer RD, Connor JH, Brown GP, Wong T, Shenolikar S, Iyengar R, Landau EM ( 1998) Gating of CaMKII by cAMP-regulated protein phosphatase activity during LTP. Science 280: 1940-1942. - PubMed
- Blitzer RD, Wong T, Nouranifar R, Iyengar R, Landau EM ( 1995) Postsynaptic cAMP pathway gates early LTP in hippocampal CA1 region. Neuron 15: 1403-1414. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous