Long-term depression of presynaptic release from the readily releasable vesicle pool induced by NMDA receptor-dependent retrograde nitric oxide - PubMed (original) (raw)
Long-term depression of presynaptic release from the readily releasable vesicle pool induced by NMDA receptor-dependent retrograde nitric oxide
Patric K Stanton et al. J Neurosci. 2003.
Abstract
Postsynaptic alterations are currently believed to be able to fully account for NMDA-receptor-dependent long-term depression (LTD) and long-term potentiation of synaptic strength, although there is also evidence supporting changes in presynaptic release. Using dualphoton laser scan microscopy of N-(3-triethylammoniumpropyl)-4-(4-(dibutylamino)styryl) pyridinium dibromide (FM1-43) to directly visualize presynaptic vesicular release at Schaffer collateral-CA1 excitatory synapses in hippocampal slices, we demonstrate reduced vesicular release associated with LTD. Selective loading, by hypertonic shock, of the readily releasable vesicle pool (RRP) showed that LTD of release is a selective modification of release from the RRP. Presynaptic LTD of RRP release required activation of NMDA receptors, production and extracellular diffusion of the intercellular messenger NO, and activation of cGMP-dependent protein kinase.
Figures
Figure 3.
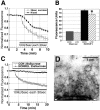
Hypertonic shock (25 sec of 800 mOsm ACSF plus sucrose) selectively loads the RRP, which represents the ∼28% of total vesicle pool brightness with more rapid release kinetics. A, Comparison of the initial 7 min of Schaffer collateral stimulus-evoked FM1-43 release time courses in 45 m
m
K +-loaded (total vesicle pool) and 25 sec 800 mOsm ACSF-loaded (RRP) slices. The first 10 Hz/5 sec stimulus released ∼12% of the RRP, compared with 2% of the total vesicle pool, confirming that sucrose preferentially loads the RRP in brain slices. B, Comparison of mean ± SEM fluorescence of Schaffer collateral terminals loaded with K + or sucrose, in control (K + = 66; sucrose = 115) versus LTD (K + = 65; sucrose = 86) puncta. *p < 0.05, Student's t test compared with control K +-loaded puncta brightness). C, Comparison of Schaffer collateral stimulus-evoked FM1-43 release time course in puncta that exhibited destaining (CON; n = 115), versus puncta that did not show stimulus-dependent destaining (NONREL; n = 15) in 25 sec 800 mOsm ACSF-loaded (RRP) slices (n = 6 slices in each group). D, Electron micrograph of a Schaffer collateral presynaptic terminal in a hippocampal slice loaded by 25 sec hyperosmotic shock and then fixed 20 min later, showing electron-dense FM1-43 reacted with 3,3′-diaminobenzidine, localized primarily to vesicles closely apposed to the release active zone (arrows).
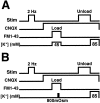
Figure 1.
Protocols for determining the kinetics of FM1-43 release from presynaptic terminals after the induction of LTD of synaptic strength. Fifteen minutes after inducing LTD, CNQX (10μ
m
) was bath-applied for 15 min, and terminals were labeled by exposure to 5μ
m
FM1-43 during either 45 m
m
K + application (15 min) to label the total vesicle pool (A), or 800 mOsm ACSF (25 sec) to label the RRP (B). Subsequent 10 Hz/5 sec bursts of electrical stimulation were applied once each 30 sec for a 20 min period to evoke the vesicular release of dye.
Figure2.
LTD of synaptic strength at Schaffer collateral–CA1 synapses produces along-lasting reduction in the evoked vesicular release of FM1-43. Two-photon excitation fluorescent images of RRP puncta in the same field in the stratum radiatum of field CA1 in a control slice (top), versus a slice in which LTD was induced (bottom), at different times after the start of the unloading stimulation protocol (numbers represent the time in minutes; 0′ is the beginning of unloading stimulation). In these slices, presynaptic vesicles in the RRP were selectively loaded with a 25 sec, 800 mOsm hypertonic ACSF plus sucrose.
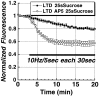
Figure 4.
LTD of synaptic strength at Schaffer collateral–CA1 synapses is associated with a selective long-lasting reduction in evoked release from the RRP. A, Time courses of Schaffer collateral stimulus-evoked (solid bar;10 Hz/5 sec bursts each 30 sec) FM1-43 destaining from the total vesicle pool in control (n = 5) versus LTD (n = 5) slices. B, Time courses of Schaffer collateral stimulus-evoked (solid bar; 10 Hz/5 sec bursts each 30 sec) FM1-43 destaining from the RRP in control (n = 7) versus LTD (n = 6) slices, illustrating the preferential reduction in release from the RRP. C, Time courses of Schaffer collateral stimulus-evoked (solid bar; 10 Hz/5 sec each 30 sec) FM1-43 release from the reserve vesicle pool in control versus LTD slices, calculated by subtracting RRP from total vesicle pool time courses. During the first 10 min, release from the reserve pool was not altered by previous induction of LTD.
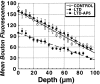
Figure 5.
LTD produces a long-lasting decrease in stimulus-evoked uptake of FM1-43 that is NMDA-receptor dependent. Comparison of _z_-axis depth profiles of mean ± SEM puncta fluorescence induced to take up FM1-43 by Schaffer collateral stimulation (10 Hz/5 sec each 30 sec for 20 min) in control slices (n = 4), versus slices in which LTD (n = 4) had been induced previously (2 Hz/10 min stimulus train), and slices in which the LTD stimulus train was applied in the presence of the NMDA receptor antagonist
d
,
l
-AP-5 (20 μ
m
; n = 4). Independent of depth, LTD was associated with decreased FM1-43 endocytosis. This decrease in FM1-43 endocytosis was completely prevented by NMDA receptor blockade.
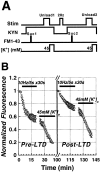
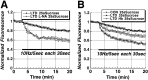
Figure 6.
The time course of Schaffer collateral stimulus-evoked (solid bars;10 Hz/5 sec bursts each 30 sec) FM1-43 release from the RRP before and after inducing LTD in the same set of slices. A, Protocol for determining the kinetics of FM1-43 release from presynaptic terminals before and during LTD in the same slices. First, kynurenic acid was bath-applied (10 m
m
KYN, 15 min), then terminals were loaded with 5 μ
m
FM1-43 in 800 mOsm sucrose ACSF for 25 sec. Release was tested 30 min later with 10 Hz/5 sec bursts of electrical stimulation each 30 sec. After residual dye was released with 45 m
m
K +, kynurenate was washed out and LTD induced (1 Hz/10 min). The identical release protocol was then repeated. B, After evoking FM1-43 release from the pre-LTD RRP (first solid bar; 10 Hz/5 sec bursts each 30 sec), 45 m
m
K + was applied to release all dye, LTD was induced, and the RRP was reloaded with FM1-43. Afterward, post-LTDFM1-43 release was evoked by a second, identical stimulus (third solid bar; 10 Hz/5 sec bursts each 30 sec). After LTD, release _t_1/2 was significantly smaller than release before LTD (p < 0.05; paired t test; n = 5).
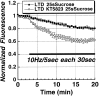
Figure 7.
LTD of vesicular release from the RRP is NMDA-receptor dependent. The time course of Schaffer collateral stimulus-evoked release from the RRP (solid bar;10 Hz/5 sec bursts each 30 sec) 45 min after the induction of LTD. Application of the NMDA receptor antagonist AP-5 (20μ
m
) 30 min before inducing LTD (n = 5) completely blocked the reduction of release, compared with control slices (n = 5).
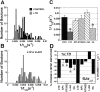
Figure 10.
Distributions of RRP puncta unloading rates (1/_t_1/2) in different groups of slices. A, Histogram of unloading rates (1/_t_1/2) of individual puncta in control slices (solid bars), versus slices in which LTD had been induced (graybars). B, Histogram of unloading rates (1/_t_1/2) of individual puncta in slices pretreated with the NMDA antagonist
d
,
l
-AP-5 (20μ
m
) 30 min before the application of the LTD stimulus. C, Mean ± SEM 1/_t_1/2(s -1) in control (solid bar), LTD (gray bar), and slices pretreated with 20 μ
m d
,
l
-AP-5 (coarsely hatched bar, left), 10 μ
m
KT5823 (finely hatched bar, left), 10 μ
m l
-NA (coarsely hatched bar, right), or 100 μ
m
Hb (finely hatched bar, right) before the application of the LTD stimulus. *p < 0.05; Student's t test compared with control 1/_t_1/2. D, Left, Mean ± SEM percentage change in fEPSP amplitude 15 min after the application of LTD-inducing stimuli (2 Hz/10 min) in the same control and drug-treated slices in which FM1-43 release was measured (*p < 0.05; Student's t test compared with control LTD); right, percentage change in 1/_t_1/2(sec -1) in the same drug conditions.
Figure 8.
LTD of vesicular release from the RRP is PKG dependent. The time course of Schaffer collateral stimulus-evoked release from the RRP (solid bar; 10 Hz/5 sec bursts each 30 sec) in slices treated with the PKG inhibitor KT5823 (10 μ
m
; n = 5) 30 min before induction of LTD, compared with control slices (n = 5).
Figure 9.
LTD of vesicular release from the RRP is NO dependent. A, Time course of Schaffer collateral stimulus-evoked release from the RRP (solid bar; 10 Hz/5 sec bursts each 30 sec) in slices treated with the NOS inhibitor
l
-NA)(10μ
m
;_n_=4)comparedwithcontrolslices(_n_=5).
l
-NA completely blocked LTD of release from the RRP. B, The NO scavenger hemoglobin (Hb; 10 μ
m
; n = 4) partially blocked the reduction in RRP release seen in LTD slices compared with control LTD (n = 5) and unstimulated slices (n = 6).
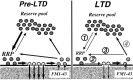
Figure 11.
Potential sites of modifications underlying the presynaptic LTD of release selectively targeting the RRP. Vesicular transmitter release targets before (Pre-LTD) and during (LTD) expression of LTD: (1) Transfer from the reserve vesicle pool to the RRP. (2) Priming and release of docked vesicles. (3) Kiss-and-run recycling of vesicles preferentially into the RRP. (4) Recycling of vesicles into the reserve pool. Although a reduction in the rates of any of these steps could produce presynaptic LTD, our observation that LTD preferentially reduces release from the RRP, without altering reserve pool size or early release kinetics suggests that the rates of RRP priming and _p_r (2), and/or re-entry (3), are reduced during LTD.
Similar articles
- Imaging LTP of presynaptic release of FM1-43 from the rapidly recycling vesicle pool of Schaffer collateral-CA1 synapses in rat hippocampal slices.
Stanton PK, Winterer J, Zhang XL, Müller W. Stanton PK, et al. Eur J Neurosci. 2005 Nov;22(10):2451-61. doi: 10.1111/j.1460-9568.2005.04437.x. Eur J Neurosci. 2005. PMID: 16307588 - FM1-43 imaging reveals cGMP-dependent long-term depression of presynaptic transmitter release.
Stanton PK, Heinemann U, Muller W. Stanton PK, et al. J Neurosci. 2001 Oct 1;21(19):RC167. doi: 10.1523/JNEUROSCI.21-19-j0002.2001. J Neurosci. 2001. PMID: 11567078 Free PMC article. - Induction and expression mechanisms of postsynaptic NMDA receptor-independent homosynaptic long-term depression.
Anwyl R. Anwyl R. Prog Neurobiol. 2006 Jan;78(1):17-37. doi: 10.1016/j.pneurobio.2005.12.001. Epub 2006 Jan 19. Prog Neurobiol. 2006. PMID: 16423442 Review. - Considerations for Measuring Activity-Dependence of Recruitment of Synaptic Vesicles to the Readily Releasable Pool.
Wesseling JF. Wesseling JF. Front Synaptic Neurosci. 2019 Nov 20;11:32. doi: 10.3389/fnsyn.2019.00032. eCollection 2019. Front Synaptic Neurosci. 2019. PMID: 31824292 Free PMC article. Review.
Cited by
- Hypothermia evoked by stimulation of medial preoptic nucleus protects the brain in a mouse model of ischaemia.
Zhang S, Zhang X, Zhong H, Li X, Wu Y, Ju J, Liu B, Zhang Z, Yan H, Wang Y, Song K, Hou ST. Zhang S, et al. Nat Commun. 2022 Nov 12;13(1):6890. doi: 10.1038/s41467-022-34735-2. Nat Commun. 2022. PMID: 36371436 Free PMC article. - The role of cGMP-dependent signaling pathway in synaptic vesicle cycle at the frog motor nerve terminals.
Petrov AM, Giniatullin AR, Sitdikova GF, Zefirov AL. Petrov AM, et al. J Neurosci. 2008 Dec 3;28(49):13216-22. doi: 10.1523/JNEUROSCI.2947-08.2008. J Neurosci. 2008. PMID: 19052213 Free PMC article. - Cortical Up states induce the selective weakening of subthreshold synaptic inputs.
Bartram J, Kahn MC, Tuohy S, Paulsen O, Wilson T, Mann EO. Bartram J, et al. Nat Commun. 2017 Sep 22;8(1):665. doi: 10.1038/s41467-017-00748-5. Nat Commun. 2017. PMID: 28939859 Free PMC article. - Phospholipase C is required for changes in postsynaptic structure and function associated with NMDA receptor-dependent long-term depression.
Horne EA, Dell'Acqua ML. Horne EA, et al. J Neurosci. 2007 Mar 28;27(13):3523-34. doi: 10.1523/JNEUROSCI.4340-06.2007. J Neurosci. 2007. PMID: 17392468 Free PMC article.
References
- Arancio O, Kandel ER, Hawkins RD ( 1995) Activity-dependent long-term enhancement of transmitter release by presynaptic 3′-5′-cGMP in cultured hippocampal neurons. Nature 376: 74-80. - PubMed
- Benke TA, Luthi A, Isaac JT, Collingridge GL ( 1998) Modulation of AMPA receptor unitary conductance by synaptic activity. Nature 393: 793-797. - PubMed
- Bekkers JM, Stevens CF ( 1990) Presynaptic mechanism for long-term potentiation in the hippocampus. Nature 346: 724-729. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous