ARF6 stimulates clathrin/AP-2 recruitment to synaptic membranes by activating phosphatidylinositol phosphate kinase type Igamma - PubMed (original) (raw)
ARF6 stimulates clathrin/AP-2 recruitment to synaptic membranes by activating phosphatidylinositol phosphate kinase type Igamma
Michael Krauss et al. J Cell Biol. 2003.
Abstract
Clathrin-mediated endocytosis of synaptic vesicle membranes involves the recruitment of clathrin and AP-2 adaptor complexes to the presynaptic plasma membrane. Phosphoinositides have been implicated in nucleating coat assembly by directly binding to several endocytotic proteins including AP-2 and AP180. Here, we show that the stimulatory effect of ATP and GTPgammaS on clathrin coat recruitment is mediated at least in part by increased levels of PIP2. We also provide evidence for a role of ADP-ribosylation factor 6 (ARF6) via direct stimulation of a synaptically enriched phosphatidylinositol 4-phosphate 5-kinase type Igamma (PIPKIgamma), in this effect. These data suggest a model according to which activation of PIPKIgamma by ARF6-GTP facilitates clathrin-coated pit assembly at the synapse.
Figures
Figure 1.
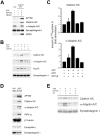
ATP/GTPγS triggers clathrin/AP-2 coat recruitment to synaptic membranes in vitro. (A) Immunoblot analysis of untreated, carbonate-washed, or LP2 membranes (6 μg) incubated with 120 μg cytosol plus ATP/GTPγS for clathrin, AP180, α-adaptin, synaptotagmin I, and ARF6. (B–E) Washed 4-μg LP2 membranes were incubated with 80 μg rat brain cytosol in the presence or absence of the indicated nucleotides. Membranes were recovered by centrifugation and analyzed by immunoblotting against clathrin heavy chain (HC), α-adaptin, AP180, Hsc70, tubulin, endophilin I, PIPKIγ, ARF6, and synaptotagmin I as a membrane marker. (B) Clathrin/AP-2 coat components associate with synaptic membranes in a nucleotide-dependent manner. (C) Quantification of the results shown in A from two independent experiments (mean ± SD). Data were normalized with respect to the amount of protein recruited in the absence of nucleotides (fold stimulation). (D) Protein recruitment in the absence of nucleotides or in the presence of ATP plus GTPγS. (E) Protein recruitment in the presence of the indicated nucleotides and with or without the broad-specificity kinase inhibitor A3.
Figure 2.
Effect of brefeldin A on clathrin/AP-2 recruitment. Clathrin/AP-2 recruitment was performed as described in Fig. 1 in the presence or absence of ATP/GTPγS and brefeldin A (BFA). Samples were analyzed by quantitative Western blot analysis using antisera against α-adaptin, AP180, synaptotagmin I as a membrane marker, and Hsc70 as a control.
Figure 3.
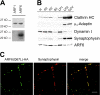
ARF6 is enriched in synaptic plasma membrane fractions. (A) Polyclonal anti-ARF6 antibodies specifically recognize ARF6, but not ARF1. Top, Ponceau-stained nitrocellulose membrane. 3 μg ARF protein was loaded per lane. Bottom, immunoblot analysis using anti-ARF6 antibodies. (B) Subcellular fractionation of pig brain homogenate according to Maycox et al. (1992). 15 μg protein was loaded per lane and analyzed by immunoblotting against clathrin heavy chain (HC), μ2-adaptin, dynamin I, synaptophysin, and ARF6. H, brain homogenate; P2, crude synaptosomes; P2′, washed synaptosomes; S3, cytosol; LP1, 20,000-g pellet after lysis of synaptosomes; LP2, 55,000-g pellet; CCV, purified clathrin-coated vesicles. (C) Localization of ARF6(Q67L) in transfected cortical neurons. Neurons at 11 days in vitro were fixed and analyzed for the distribution of HA-tagged ARF6(Q67L) and the presynaptic marker protein synaptophysin using a Leica confocal laser microscope. Bar, 10 μm. Note that synaptophysin-positive synapses devoid of ARF6(Q67L) may originate from nontransfected neurons.
Figure 4.
ARF6-GTP stimulates clathrin/AP-2 recruitment to synaptic membranes. (A) Coat recruitment to LP2 membranes was performed and analyzed as described in the legend to Fig. 1. Samples containing 1-μM ARF6 mutants were incubated in the presence of 200 μM GTP and analyzed by quantitative immunoblotting against clathrin heavy chain (HC), AP180, α-adaptin, Hsc70, and synaptotagmin I. (B) Dose dependence of the stimulatory effect of ARF6(Q67L) on clathrin recruitment to membranes as shown in A. Values were normalized to the amount of clathrin bound in the presence of ATP and GTPγS (100%). (C) Dose dependence of the inhibitory effect of ARF6(T27N) on clathrin recruitment to membranes as shown in A. Values were normalized to the amount of clathrin bound in the presence of ATP and GTPγS (100%).
Figure 5.
Activated ARF6 interacts with PIPKIγ in brain. (A) ARF6(Q67L) but not ARF6(T27N) specifically affinity purifies PIPKIγ. Western blot analysis of proteins pulled down by GST and GST–ARF6 fusion proteins (80 μg) from a detergent extract of rat brain. Samples were analyzed by SDS-PAGE and immunoblotting. 5% Std., 5% of the extract used for affinity purification. (B) PIPKIγ specifically interacts with ARF6(Q67L). Immunoblot analysis of PIPKIγ affinity purified with myristoylated His6-tagged ARF6(Q67L), ARF1(Q71L), or arfaptin 2 as described under A. 5% Std., 5% of the extract used for affinity purification. (C) PIPKIγ can be cross-linked to ARF6(Q67L) during recruitment of clathrin/AP-2 to synaptic membranes. LP2-membranes were incubated with brain cytosol, myristoylated His6-tagged ARF6, and nucleotides. DTSP was added where indicated. His6-tagged ARF6 was recovered and cross-linked proteins were analyzed by immunoblotting. Top, immunoblot analysis with antisera against PIPKIγ, AP180, large and medium subunits of the AP-2 complex β1/2-adaptin, α-adaptin, and μ2-adaptin, respectively, and clathrin heavy chain (HC). Bottom, Coomassie-stained gel demonstrating that equal amounts of ARF6 have been recovered in each sample.
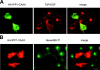
Figure 6.
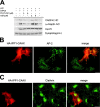
ARF6(Q67L) colocalizes with PIPKIγ in transfected cells. Cos7 cells were cotransfected with plasmids encoding ARF6(Q67L)-EGFP (A), ARF6(T27N)-EGFP (B), or PHPLCδ-EGFP (C) and HA-tagged PIPKIγ. 24 h after transfection, the cells were fixed and analyzed by immunofluorescence microscopy. Merged images are shown in A–C (middle panels). Bar, 20 μm.
Figure 7.
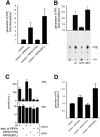
ARF6 directly stimulates the activity of endogenous or recombinant PIPKIγ. (A) Liposomes containing phosphatidylinositol 4-phosphate (6%, wt/wt) as a substrate were incubated for 10 min at 37°C with brain cytosol and recombinant myristoylated ARF6 mutants (200 nM) as indicated in the presence of neomycin, GTP, and γ[32P]ATP. Lipids were extracted and separated by HPTLC. Data represent mean values (± SD) from four independent experiments. Values were normalized to the amount of PIP2 generated in the absence of ARF6 proteins (fold stimulation). (B) 4 ng recombinant PIPKIγ was pre-incubated for 20 min at 4°C with recombinant myristoylated ARF6 proteins, GTP, and total brain liposomes. Reactions were started by addition of γ[32P]ATP, and after a 7-min incubation (37°C), lipid products were extracted, separated by TLC as described previously (Wenk et al., 2001), analyzed by autoradiography (see bottom for a typical experiment) and quantified (Phosphor- Imager). The top shows the quantification of three independent experiments (mean ± SD); the data presented are normalized to the activity of PIPKIγ in the absence of exogenously added ARF6 proteins (fold stimulation). (C) 100 μg rat brain cytosol that had either been mock-depleted (ctrl) or depleted of PIPKIγ was pre-incubated (20 min at 4°C) with myristoylated ARF6 proteins, GTP, and total brain liposomes. The reaction (7 min at 37°C) was started by addition of γ[32P]ATP. Samples were analyzed as described above. Formation of PIP2 and PIP is depicted as mean ± SD (n = 3). (D) Brain cytosol was pre-incubated (2 min at 37°C) with GTP, γ[32P]ATP, neomycin, and recombinant ARF6 protein as indicated. Reactions (15 min at 37°C) were started by addition of LP2 membranes. Samples were analyzed as described under A. Data are depicted as mean (± SD) from four independent experiments.
Figure 8.
Effects of PIP2 masking or degradation on clathrin/AP-2–coated pit assembly. (A) Recombinant human PHPLCδ1 inhibits ATP/GTPγS-induced membrane recruitment of clathrin/AP-2 coat components. Clathrin/AP-2 recruitment onto presynaptic membranes was performed as described in Fig. 1 in the presence or absence of ATP/GTPγS, purified PHPLCδ1, or BSA. Samples were analyzed by quantitative Western blotting using antisera against clathrin heavy chain (HC), α-adaptin, synaptotagmin I as a membrane marker, and Hsc70 as a control. (B and C) Overexpression of membrane-targeted HA-tagged inositol 5-phosphate phosphatase domain of synaptojanin 1 (HA-IPP1-CAAX) mislocalizes clathrin and AP-2. Cos7 cells expressing HA-IPP1-CAAX were fixed 24 h after transfection and analyzed for the distribution of AP-2 (B) or clathrin (C) by immunofluorescence microscopy. Bar, 20 μm.
Figure 9.
PIP2 degradation inhibits receptor-mediated internalization of EGF and transferrin. Overexpression of membrane-targeted HA-tagged inositol phosphatase domain of synaptojanin 1 (HA-IPP1-CAAX) inhibits the internalization of epidermal growth factor (A; EGF) or transferrin (B; Tf). Cos7 cells expressing HA-IPP1-CAAX were allowed to internalize Texas red–labeled epidermal growth factor (A) or Alexa® 488-transferrin (B) for 10 min at 37°C. Cells were fixed and analyzed by immunofluorescence microscopy. Bar, 20 μm.
Similar articles
- Functional assay of effectors of ADP ribosylation factor 6 during clathrin/AP-2 coat recruitment to membranes.
Krauss M, Haucke V. Krauss M, et al. Methods Enzymol. 2005;404:388-98. doi: 10.1016/S0076-6879(05)04034-6. Methods Enzymol. 2005. PMID: 16413285 - ARF6 regulates angiotensin II type 1 receptor endocytosis by controlling the recruitment of AP-2 and clathrin.
Poupart ME, Fessart D, Cotton M, Laporte SA, Claing A. Poupart ME, et al. Cell Signal. 2007 Nov;19(11):2370-8. doi: 10.1016/j.cellsig.2007.07.015. Epub 2007 Jul 31. Cell Signal. 2007. PMID: 17719203 - PIP kinase Igamma is the major PI(4,5)P(2) synthesizing enzyme at the synapse.
Wenk MR, Pellegrini L, Klenchin VA, Di Paolo G, Chang S, Daniell L, Arioka M, Martin TF, De Camilli P. Wenk MR, et al. Neuron. 2001 Oct 11;32(1):79-88. doi: 10.1016/s0896-6273(01)00456-1. Neuron. 2001. PMID: 11604140 - Sorting it out: AP-2 and alternate clathrin adaptors in endocytic cargo selection.
Traub LM. Traub LM. J Cell Biol. 2003 Oct 27;163(2):203-8. doi: 10.1083/jcb.200309175. J Cell Biol. 2003. PMID: 14581447 Free PMC article. Review. - Clathrin and synaptic vesicle endocytosis: studies at the squid giant synapse.
Augustine GJ, Morgan JR, Villalba-Galea CA, Jin S, Prasad K, Lafer EM. Augustine GJ, et al. Biochem Soc Trans. 2006 Feb;34(Pt 1):68-72. doi: 10.1042/BST0340068. Biochem Soc Trans. 2006. PMID: 16417485 Free PMC article. Review.
Cited by
- Inositol 5-phosphatases: insights from the Lowe syndrome protein OCRL.
Pirruccello M, De Camilli P. Pirruccello M, et al. Trends Biochem Sci. 2012 Apr;37(4):134-43. doi: 10.1016/j.tibs.2012.01.002. Epub 2012 Feb 28. Trends Biochem Sci. 2012. PMID: 22381590 Free PMC article. Review. - Role of activation of PIP5Kgamma661 by AP-2 complex in synaptic vesicle endocytosis.
Nakano-Kobayashi A, Yamazaki M, Unoki T, Hongu T, Murata C, Taguchi R, Katada T, Frohman MA, Yokozeki T, Kanaho Y. Nakano-Kobayashi A, et al. EMBO J. 2007 Feb 21;26(4):1105-16. doi: 10.1038/sj.emboj.7601573. Epub 2007 Feb 8. EMBO J. 2007. PMID: 17290217 Free PMC article. - Arf6 Regulates Endocytosis and Angiogenesis by Promoting Filamentous Actin Assembly.
Francis CR, Bell ML, Skripnichuk MM, Kushner EJ. Francis CR, et al. bioRxiv [Preprint]. 2023 Feb 22:2023.02.22.529543. doi: 10.1101/2023.02.22.529543. bioRxiv. 2023. PMID: 36865161 Free PMC article. Updated. Preprint. - An effector domain mutant of Arf6 implicates phospholipase D in endosomal membrane recycling.
Jovanovic OA, Brown FD, Donaldson JG. Jovanovic OA, et al. Mol Biol Cell. 2006 Jan;17(1):327-35. doi: 10.1091/mbc.e05-06-0523. Epub 2005 Nov 9. Mol Biol Cell. 2006. PMID: 16280360 Free PMC article. - TBC1D24, an ARF6-interacting protein, is mutated in familial infantile myoclonic epilepsy.
Falace A, Filipello F, La Padula V, Vanni N, Madia F, De Pietri Tonelli D, de Falco FA, Striano P, Dagna Bricarelli F, Minetti C, Benfenati F, Fassio A, Zara F. Falace A, et al. Am J Hum Genet. 2010 Sep 10;87(3):365-70. doi: 10.1016/j.ajhg.2010.07.020. Epub 2010 Aug 19. Am J Hum Genet. 2010. PMID: 20727515 Free PMC article.
References
- Arneson, L.S., J. Kunz, R.A. Anderson, and L.M. Traub. 1999. Coupled inositide phosphorylation and phospholipase D activation initiates clathrin-coat assembly on lysosomes. J. Biol. Chem. 274:17794–17805. - PubMed
- Austin, C., M. Boehm, and S.A. Tooze. 2002. Site-specific cross-linking reveals a differential direct interaction of class 1, 2, and 3 ADP-ribosylation factors with adaptor protein complexes 1 and 3. Biochemistry. 41:4669–4677. - PubMed
- Barbieri, M.A., C.M. Heath, E.M. Peters, A. Wells, J.N. Davis, and P.D. Stahl. 2001. Phosphatidylinositol 4-phosphate 5-kinase Iβ is essential for epidermal growth factor receptor-mediated endocytosis. J. Biol. Chem. 276:47212–47216. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials