Bacteria-host communication: the language of hormones - PubMed (original) (raw)
Bacteria-host communication: the language of hormones
Vanessa Sperandio et al. Proc Natl Acad Sci U S A. 2003.
Abstract
The interbacterial communication system known as quorum sensing (QS) utilizes hormone-like compounds referred to as autoinducers to regulate bacterial gene expression. Enterohemorrhagic Escherichia coli (EHEC) serotype O157:H7 is the agent responsible for outbreaks of bloody diarrhea in several countries. We previously proposed that EHEC uses a QS regulatory system to "sense" that it is within the intestine and activate genes essential for intestinal colonization. The QS system used by EHEC is the LuxS/autoinducer 2 (AI-2) system extensively involved in interspecies communication. The autoinducer AI-2 is a furanosyl borate diester whose synthesis depends on the enzyme LuxS. Here we show that an EHEC luxS mutant, unable to produce the bacterial autoinducer, still responds to a eukaryotic cell signal to activate expression of its virulence genes. We have identified this signal as the hormone epinephrine and show that beta- and alpha-adrenergic antagonists can block the bacterial response to this hormone. Furthermore, using purified and in vitro synthesized AI-2 we showed that AI-2 is not the autoinducer involved in the bacterial signaling. EHEC produces another, previously undescribed autoinducer (AI-3) whose synthesis depends on the presence of LuxS. These results imply a potential cross-communication between the luxS/AI-3 bacterial QS system and the epinephrine host signaling system. Given that eukaryotic cell-to-cell signaling typically occurs through hormones, and that bacterial cell-to-cell signaling occurs through QS, we speculate that QS might be a "language" by which bacteria and host cells communicate.
Figures
Fig. 1.
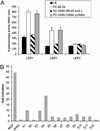
(A) Transcription of LEE::lacZ fusions integrated into the K-12 chromosome in fresh medium (LB) and medium preconditioned by growth of WT strain 86-24, the luxS mutant (VS94), or the complemented mutant (VS95). (B) Induction of luminescence in_V. harveyi_ strain BB170 by fecal filtrates from volunteers from the Center for Vaccine Development at the University of Maryland School of Medicine. As positive and negative controls we used PC medium with 86-24 and DH5α (which does not produce AI-2), respectively.
Fig. 2.
(A) Western blot of type III-secreted proteins from strains 86-24, VS94, and VS95 in fresh DMEM; secreted proteins from VS94 in DMEM preconditioned with HeLa cells, nonpreconditioned DMEM + 10% FBS, DMEM + 50 μM of Epi (E), or 50 μM of NE. (B) β-galactosidase activity of a LEE1::lacZ chromosomal fusion in K-12 grown in fresh DMEM to an OD600 ≤ 0.2 in the presence of 50 μM Epi, NE, PO, PE, gastrin (GA), galanin (GL), and secretin (S) or with no additives (M). (C) Western blot of secreted proteins from VS94 and 86-24 grown in fresh DMEM (M), DMEM + 50 μM Epi, DMEM + 500 μM PO, DMEM + 50 μM Epi and 500 μM PO (E/PO), DMEM + 500 μM PE, and DMEM + 50 μM Epi and 500 μM PE (E/PE). (D) Western blot of flagellin from 86-24, VS94, VS95, VS94 + PC medium with HeLa cells, and VS94 + 50 μM of Epi. (E) Motility in DMEM + 50 μM Epi of EHEC 86-24, luxS (VS94), and qseC (VS138) mutants. (F) Transcription of_qseA_::lacZ in fresh medium (M) and in DMEM + 50 μM of Epi, PE, and PO in WT and _luxS_– backgrounds.*, Transcription in the luxS mutant with PE and PO was performed in the presence of Epi; no Epi was added to the WT strain.
Fig. 3.
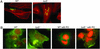
(A) Formation of AE lesions, by using the fluorescein actin staining (FAS) test, by WT and luxS mutant in HeLa cells. The actin cytoskeleton is stained in red with Alexa-phalloidin, and EHEC is stained in green with anti-O157 antiserum conjugated with FITC. (B) FAS test of the WT and luxS mutant without and with PO (500 μM). The actin cytoskeleton is stained in green with FITC-phalloidin, and EHEC is stained in red with propidium iodide.
Fig. 4.
(A) V. harveyi AI-2 luminescence assay in the presence of the purified fractions containing AI-2 (AI-2F) and AI-3 (AI-3F), in vitro synthesized AI-2 (AI-2S) (10 and 100 μM), and PC medium prepared with 86-24 (positive control), DH5α, and VS94. (B) Transcription of LEE1::lacZ in fresh medium (M), in the presence of the purified fractions AI-2 and AI-3, and in in vitro synthesized AI-2. (C) Western blot of secreted proteins from strain 86-24, VS94, VS94 + 100 μM of AI-2S, VS94 + 50 μM of Epi (Sigma), and VS94 + 4 μM of AI-3. (D) Electrospray mass spectrometry of the AI-3 purified fraction. (E) Transcription of_LEE1_::lacZ in fresh medium (M), in the presence of AI-3 (4 μM), and in AI-2S (100 μM). (F) Transcription of_LEE1_::lacZ in fresh medium (LB), PC medium with strain 86-24 (PC-WT), PC medium with strain VS94 (PC-luxS), and PC medium with an E. coli entA mutant (PC-entA). (G) Transcription of_qseBC_::lacZ in a luxS mutant in the presence of AI-3 (4 μM) and AI-2S (100 μM). (H) Transcription of_flhDC_::lacZ in a luxS or qseC mutant background in the presence of AI-3 (4 μM) or Epi (E; 50 μM).
Similar articles
- Global effects of the cell-to-cell signaling molecules autoinducer-2, autoinducer-3, and epinephrine in a luxS mutant of enterohemorrhagic Escherichia coli.
Kendall MM, Rasko DA, Sperandio V. Kendall MM, et al. Infect Immun. 2007 Oct;75(10):4875-84. doi: 10.1128/IAI.00550-07. Epub 2007 Jul 16. Infect Immun. 2007. PMID: 17635870 Free PMC article. - Autoinducer 3 and epinephrine signaling in the kinetics of locus of enterocyte effacement gene expression in enterohemorrhagic Escherichia coli.
Walters M, Sperandio V. Walters M, et al. Infect Immun. 2006 Oct;74(10):5445-55. doi: 10.1128/IAI.00099-06. Infect Immun. 2006. PMID: 16988219 Free PMC article. - AI-3 synthesis is not dependent on luxS in Escherichia coli.
Walters M, Sircili MP, Sperandio V. Walters M, et al. J Bacteriol. 2006 Aug;188(16):5668-81. doi: 10.1128/JB.00648-06. J Bacteriol. 2006. PMID: 16885435 Free PMC article. - SdiA sensing of acyl-homoserine lactones by enterohemorrhagic E. coli (EHEC) serotype O157:H7 in the bovine rumen.
Sperandio V. Sperandio V. Gut Microbes. 2010 Nov-Dec;1(6):432-5. doi: 10.4161/gmic.1.6.14177. Gut Microbes. 2010. PMID: 21468228 Free PMC article. Review. - Quorum sensing and expression of virulence in Escherichia coli O157:H7.
Anand SK, Griffiths MW. Anand SK, et al. Int J Food Microbiol. 2003 Aug 15;85(1-2):1-9. doi: 10.1016/s0168-1605(02)00482-8. Int J Food Microbiol. 2003. PMID: 12810266 Review.
Cited by
- Colonizing foreign terrain: Insights into bacterial enteropathogens.
Kattner AA. Kattner AA. Biomed J. 2023 Nov 30;46(6):100681. doi: 10.1016/j.bj.2023.100681. Online ahead of print. Biomed J. 2023. PMID: 38042347 Free PMC article. - Regulatory Mechanisms between Quorum Sensing and Virulence in Salmonella.
Zhang X, Liu B, Ding X, Bin P, Yang Y, Zhu G. Zhang X, et al. Microorganisms. 2022 Nov 9;10(11):2211. doi: 10.3390/microorganisms10112211. Microorganisms. 2022. PMID: 36363803 Free PMC article. Review. - Bundle-forming pili and EspA are involved in biofilm formation by enteropathogenic Escherichia coli.
Moreira CG, Palmer K, Whiteley M, Sircili MP, Trabulsi LR, Castro AF, Sperandio V. Moreira CG, et al. J Bacteriol. 2006 Jun;188(11):3952-61. doi: 10.1128/JB.00177-06. J Bacteriol. 2006. PMID: 16707687 Free PMC article. - The Role of Quorum Sensing Molecules in Bacterial-Plant Interactions.
Majdura J, Jankiewicz U, Gałązka A, Orzechowski S. Majdura J, et al. Metabolites. 2023 Jan 10;13(1):114. doi: 10.3390/metabo13010114. Metabolites. 2023. PMID: 36677039 Free PMC article. Review. - Medicinal chemistry as a conduit for the modulation of quorum sensing.
Lowery CA, Salzameda NT, Sawada D, Kaufmann GF, Janda KD. Lowery CA, et al. J Med Chem. 2010 Nov 11;53(21):7467-89. doi: 10.1021/jm901742e. J Med Chem. 2010. PMID: 20669927 Free PMC article. No abstract available.
References
- Kaper, J. B. & O'Brien, A. D. (1998) Escherichia coli O157:H7 and Other Shiga Toxin-Producing E. coli Strains (Am. Soc. Microbiol., Washington, DC). - PubMed
- Elliott, S. J., Wainwright, L. A., McDaniel, T. K., Jarvis, K. G., Deng, Y. K., Lai, L. C., McNamara, B. P., Donnenberg, M. S. & Kaper, J. B. (1998) Mol. Microbiol. 28, 1–4. - PubMed
- Mellies, J. L., Elliott, S. J., Sperandio, V., Donnenberg, M. S. & Kaper, J. B. (1999) Mol. Microbiol. 33, 296–306. - PubMed
- Sperandio, V., Mellies, J. L., Delahay, R. M., Frankel, G., Crawford, J. A., Nguyen, W. & Kaper, J. B. (2000) Mol. Microbiol. 38, 781–793. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources