Possible involvement of P2Y2 metabotropic receptors in ATP-induced transient receptor potential vanilloid receptor 1-mediated thermal hypersensitivity - PubMed (original) (raw)
. 2003 Jul 9;23(14):6058-62.
doi: 10.1523/JNEUROSCI.23-14-06058.2003.
Tohko Iida, Kimiko Kobayashi, Tomohiro Higashi, Tetsuo Fukuoka, Hideki Tsumura, Catherine Leon, Noboru Suzuki, Kazuhide Inoue, Christian Gachet, Koichi Noguchi, Makoto Tominaga
Affiliations
- PMID: 12853424
- PMCID: PMC6740351
- DOI: 10.1523/JNEUROSCI.23-14-06058.2003
Possible involvement of P2Y2 metabotropic receptors in ATP-induced transient receptor potential vanilloid receptor 1-mediated thermal hypersensitivity
Tomoko Moriyama et al. J Neurosci. 2003.
Abstract
The capsaicin receptor transient receptor potential V1 (TRPV1; also known as vanilloid receptor 1) is a sensory neuron-specific ion channel that serves as a polymodal detector of pain-producing chemical and physical stimuli. It has been reported that extracellular ATP potentiates the TRPV1 currents evoked by capsaicin or protons and reduces the temperature threshold for its activation through metabotropic P2Y receptors in a PKC-dependent pathway, suggesting that TRPV1 activation could trigger the sensation of pain at normal body temperature in the presence of ATP. Here, we show that ATP-induced thermal hyperalgesia was abolished in mice lacking TRPV1, suggesting the functional interaction between ATP and TRPV1 at a behavioral level. However, thermal hyperalgesia was preserved in P2Y1 receptor-deficient mice. Patch-clamp analyses using mouse dorsal root ganglion neurons indicated the involvement of P2Y2 rather than P2Y1 receptors. Coexpression of TRPV1 mRNA with P2Y2 mRNA, but not P2Y1 mRNA, was determined in the rat lumbar DRG using in situ hybridization histochemistry. These data indicate the importance of metabotropic P2Y2 receptors in nociception through TRPV1.
Figures
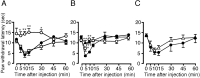
Figure 1.
TRPV1 is essential for the development of ATP-induced thermal hypersensitivity in vivo, and P2Y receptors are predominantly involved. A, Wild-type (•), TRPV1 -/- (○), or P2Y1-/- mice (□) were injected intraplantarly with ATP (100 nmol), and the response latency to radiant heating of the hindpaw was measured at various time points after injection. Values are expressed as mean ± SE; n = 6 for each group. *p < 0.05 and **p < 0.01 versus wild-type and P2Y1-/- mice; two-tailed unpaired t test. B, Behavioral analyses in response to αβ meATP (20 nmol) injection similar to A in wild-type (•,▴) or TRPV1 -/- mice (○). In some experiments, mice were pretreated with TNP–ATP (50 nmol) (▴). Values are expressed as mean ± SE; n = 6 for each group. *p < 0.05 and **p < 0.01 versus wild type without TNP–ATP pretreatment; two-tailed unpaired t test. C, Behavioral analyses in response to ATP (100 nmol) injection similar to A in wild-type mice pretreated with TNP–ATP (50 nmol) (▵) or saline (•). Values are expressed as mean ± SE; n = 6 for each group.
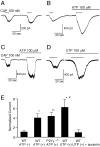
Figure 2.
Extracellular ATP or UTP potentiates capsaicin-evoked currents in DRG neurons from wild-type or P2Y1-deficient mice. A–C, Representative traces of increases in the capsaicin (CAP)-activated currents by extracellular ATP (100 μ
m
) in DRG neurons from wild-type (WT) (B) or P2Y1-deficient (C) mice. A shows a control trace without ATP pretreatment. Holding potential was -60 mV. D, A representative trace of increases in the capsaicin-activated currents by UTP (100 μ
m
) in DRG neurons from wild-type mice. E, Effects of ATP, UTP, or UTP plus suramin (50 μ
m
) on the capsaicin-activated currents. Currents were normalized to the currents initially evoked by capsaicin (100 n
m
) in the absence of the additives. Normalized currents in the absence of ATP in wild-type mice, in the presence of ATP in wild-type mice, in the presence of ATP in P2Y1-deficient mice, in the presence of UTP in wild-type mice, or in the presence of UTP plus suramin in wild-type mice were 1.07 ± 0.26 (n = 3), 4.01 ± 0.92 (n = 8), 4.37 ± 0.74 (n = 4), 6.24±1.58 (n = 4), or 0.95 ± 0.57 (n = 3), respectively. *p < 0.05 and **p < 0.01 versus absence of ATP in wild type; #p < 0.05 versus presence of UTP and suramin in wild type; two-tailed unpaired t test. WT, Wild type.
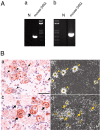
Figure 3.
P2Y2 mRNA, but not P2Y1 mRNA, is coexpressed with TRPV1 mRNA in DRG. A, PCR amplification of P2Y1 (a) and P2Y2 (b) cDNA fragments from the RNAs of mouse DRG neurons. The expected sizes of the DNA fragments for mouse P2Y1 and P2Y2 are 651 and 781 bp, respectively. N, Negative control. B, Coexpression of TRPV1 mRNA with P2Y1 and P2Y2 mRNAs in rat DRG neurons. Double in situ hybridization histochemistry was performed on the sections. b and d are dark-field photomicrographs of a and c, respectively. The TRPV1 mRNA-expressing neurons were hybridized by a DIG-labeled antisense probe and visualized as DAB staining (brown cells in a and c). The P2Y1 mRNA-expressing (b) and P2Y2 mRNA-expressing (d) neurons were hybridized by the appropriate 35S-labeled antisense probe and visualized as clusters of silver grains. p, Positively labeled neurons. Arrowheads indicate P2Y1 mRNA-expressing neurons that do not express TRPV1 mRNA, whereas arrows indicate the neurons expressing both TRPV1 and P2Y2 mRNAs.
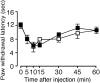
Figure 4.
UTP induces thermal hyperalgesia in mice. Behavioral analyses in response to UTP (100 nmol) injection similar to Figure 1 in wild-type mice pretreated with TNP–ATP (50 nmol) (□) or saline (•). Values are expressed as mean ± SE; n = 6 for each group.
Similar articles
- Thermal nociception and TRPV1 function are attenuated in mice lacking the nucleotide receptor P2Y2.
Malin SA, Davis BM, Koerber RH, Reynolds IJ, Albers KM, Molliver DC. Malin SA, et al. Pain. 2008 Sep 15;138(3):484-496. doi: 10.1016/j.pain.2008.01.026. Epub 2008 Mar 14. Pain. 2008. PMID: 18343036 Free PMC article. - P2Y2 receptors mediate ATP-induced resensitization of TRPV1 expressed by kidney projecting sensory neurons.
Wang H, Wang DH, Galligan JJ. Wang H, et al. Am J Physiol Regul Integr Comp Physiol. 2010 Jun;298(6):R1634-41. doi: 10.1152/ajpregu.00235.2009. Epub 2010 Mar 24. Am J Physiol Regul Integr Comp Physiol. 2010. PMID: 20335377 Free PMC article. - Co-activation of P2Y2 receptor and TRPV channel by ATP: implications for ATP induced pain.
Lakshmi S, Joshi PG. Lakshmi S, et al. Cell Mol Neurobiol. 2005 Aug;25(5):819-32. doi: 10.1007/s10571-005-4936-8. Cell Mol Neurobiol. 2005. PMID: 16133936 Free PMC article. - [Molecular mechanisms of nociception].
Tominaga M, Numazaki M, Iida T, Tominaga T. Tominaga M, et al. Nihon Shinkei Seishin Yakurigaku Zasshi. 2003 Jun;23(3):139-47. Nihon Shinkei Seishin Yakurigaku Zasshi. 2003. PMID: 12884755 Review. Japanese.
Cited by
- Activation of TRPV1 by capsaicin induces functional kinin B(1) receptor in rat spinal cord microglia.
Talbot S, Dias JP, Lahjouji K, Bogo MR, Campos MM, Gaudreau P, Couture R. Talbot S, et al. J Neuroinflammation. 2012 Jan 20;9:16. doi: 10.1186/1742-2094-9-16. J Neuroinflammation. 2012. PMID: 22264228 Free PMC article. - P2Y Receptors in Synaptic Transmission and Plasticity: Therapeutic Potential in Cognitive Dysfunction.
Guzman SJ, Gerevich Z. Guzman SJ, et al. Neural Plast. 2016;2016:1207393. doi: 10.1155/2016/1207393. Epub 2016 Mar 16. Neural Plast. 2016. PMID: 27069691 Free PMC article. Review. - Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins.
Moriyama T, Higashi T, Togashi K, Iida T, Segi E, Sugimoto Y, Tominaga T, Narumiya S, Tominaga M. Moriyama T, et al. Mol Pain. 2005 Jan 17;1:3. doi: 10.1186/1744-8069-1-3. Mol Pain. 2005. PMID: 15813989 Free PMC article. - Regulation of neuronal ion channels via P2Y receptors.
Lechner SG, Boehm S. Lechner SG, et al. Purinergic Signal. 2004 Dec;1(1):31-41. doi: 10.1007/s11302-004-4746-3. Purinergic Signal. 2004. PMID: 18404398 Free PMC article. - Protein kinase C in pain: involvement of multiple isoforms.
Velázquez KT, Mohammad H, Sweitzer SM. Velázquez KT, et al. Pharmacol Res. 2007 Jun;55(6):578-89. doi: 10.1016/j.phrs.2007.04.006. Epub 2007 Apr 29. Pharmacol Res. 2007. PMID: 17548207 Free PMC article. Review.
References
- Anderson CM, Parkinson FE ( 1997) Potential signaling roles for UTP and UDP: sources, regulation and release of uracil nucleotides. Trends Pharmacol Sci 18: 387–392. - PubMed
- Caterina MJ, Julius D ( 2001) The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci 24: 487–517. - PubMed
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D ( 1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824. - PubMed
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D ( 2000) Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288: 306–313. - PubMed
- Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN ( 1995) A P2X purinoceptor expressed by a subset of sensory neurons. Nature 377: 428–431. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases