Tau filaments from human brain and from in vitro assembly of recombinant protein show cross-beta structure - PubMed (original) (raw)
Tau filaments from human brain and from in vitro assembly of recombinant protein show cross-beta structure
John Berriman et al. Proc Natl Acad Sci U S A. 2003.
Abstract
Abnormal filaments consisting of hyperphosphorylated microtubule-associated protein tau form in the brains of patients with Alzheimer's disease, Down's syndrome, and various dementing tauopathies. In Alzheimer's disease and Down's syndrome, the filaments have two characteristic morphologies referred to as paired helical and straight filaments, whereas in tauopathies, there is a wider range of morphologies. There has been controversy in the literature concerning the internal molecular fine structure of these filaments, with arguments for and against the cross-beta structure demonstrated in many other amyloid fibers. The difficulty is to produce from brain pure preparations of filaments for analysis. One approach to avoid the need for a pure preparation is to use selected area electron diffraction from small groups of filaments of defined morphology. Alternatively, it is possible to assemble filaments in vitro from expressed tau protein to produce a homogeneous specimen suitable for analysis by electron diffraction, x-ray diffraction, and Fourier transform infrared spectroscopy. Using both these approaches, we show here that native filaments from brain and filaments assembled in vitro from expressed tau protein have a clear cross-beta structure.
Figures
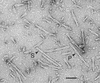
Fig. 1.
Electron micrograph of a negatively stained preparation of abnormal tau-containing filaments from the frontal cortex of a patient with Down's syndrome. Examples of the characteristic PHF (P) and SF (S) are indicated. (Bar = 100 nm.)
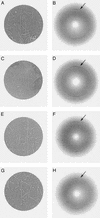
Fig. 2.
Selected area electron diffraction patterns from an unstained frozen preparation of tau filaments from a patient with Down's syndrome. A, C, E, and G show the areas from which the corresponding diffraction patterns B, D, F, and H were recorded. In A, the clump of filaments is partially dehydrated but shows clearly the morphology of PHFs. In C, the filaments are fully embedded in ice. In E, the scattering arises predominantly from one PHF and in G from one SF. The diffraction patterns (B, D, F, and H), which have been aligned relative to the images (A, C, E, and G) and which have had the strong central scattering removed, all show a clear arc at 0.47-nm spacing (arrowed) in the axial direction of the filaments, indicative of cross-β structure.
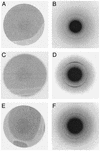
Fig. 3.
Selected area electron diffraction patterns from tau filaments assembled_in vitro. A, C_, and E show images of areas from which diffraction patterns B, D, and F were recorded. (A and B) Wild-type four-repeat human tau. (C and D) P301S mutant 4-repeat tau. (E and F) K257T mutant three-repeat tau. The diffraction patterns show strong scattering in the 0.47-nm region with partial orientation indicative of cross-β structure.
Fig. 4.
X-ray diffraction pattern from a partially aligned fiber of tau filaments assembled in vitro. The filaments were assembled from P301S mutant four-repeat human tau. There is strong meridional scattering at a spacing of 0.47-nm and a weak ring, shown with enhanced contrast in the central inset, at a spacing of 1.3 nm.
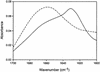
Fig. 5.
FTIR spectra of soluble and filamentous P301S mutant four-repeat human tau. The spectrum of soluble tau (dashed line) shows no indication of β-structure, but the filamentous sample (solid line) shows a strong peak at 1,628 cm-1, indicating that the assembled filaments have significant β-structure.
Similar articles
- Tau paired helical filaments from Alzheimer's disease brain and assembled in vitro are based on beta-structure in the core domain.
Barghorn S, Davies P, Mandelkow E. Barghorn S, et al. Biochemistry. 2004 Feb 17;43(6):1694-703. doi: 10.1021/bi0357006. Biochemistry. 2004. PMID: 14769047 - Protein anatomy: C-tail region of human tau protein as a crucial structural element in Alzheimer's paired helical filament formation in vitro.
Yanagawa H, Chung SH, Ogawa Y, Sato K, Shibata-Seki T, Masai J, Ishiguro K. Yanagawa H, et al. Biochemistry. 1998 Feb 17;37(7):1979-88. doi: 10.1021/bi9724265. Biochemistry. 1998. PMID: 9485325 - Positional effects of phosphorylation on the stability and morphology of tau-related amyloid fibrils.
Inoue M, Konno T, Tainaka K, Nakata E, Yoshida HO, Morii T. Inoue M, et al. Biochemistry. 2012 Feb 21;51(7):1396-406. doi: 10.1021/bi201451z. Epub 2012 Feb 7. Biochemistry. 2012. PMID: 22304362 - Abnormal tau-containing filaments in neurodegenerative diseases.
Crowther RA, Goedert M. Crowther RA, et al. J Struct Biol. 2000 Jun;130(2-3):271-9. doi: 10.1006/jsbi.2000.4270. J Struct Biol. 2000. PMID: 10940231 Review. - Tau aggregation is driven by a transition from random coil to beta sheet structure.
von Bergen M, Barghorn S, Biernat J, Mandelkow EM, Mandelkow E. von Bergen M, et al. Biochim Biophys Acta. 2005 Jan 3;1739(2-3):158-66. doi: 10.1016/j.bbadis.2004.09.010. Epub 2004 Nov 12. Biochim Biophys Acta. 2005. PMID: 15615635 Review.
Cited by
- Asparagine residue 368 is involved in Alzheimer's disease tau strain-specific aggregation.
Shimonaka S, Matsumoto SE, Elahi M, Ishiguro K, Hasegawa M, Hattori N, Motoi Y. Shimonaka S, et al. J Biol Chem. 2020 Oct 9;295(41):13996-14014. doi: 10.1074/jbc.RA120.013271. Epub 2020 Aug 5. J Biol Chem. 2020. PMID: 32759167 Free PMC article. - Structural transitions in tau k18 on micelle binding suggest a hierarchy in the efficacy of individual microtubule-binding repeats in filament nucleation.
Barré P, Eliezer D. Barré P, et al. Protein Sci. 2013 Aug;22(8):1037-48. doi: 10.1002/pro.2290. Epub 2013 Jun 24. Protein Sci. 2013. PMID: 23740819 Free PMC article. - Tau protein in familial and sporadic diseases.
Yancopoulou D, Spillantini MG. Yancopoulou D, et al. Neuromolecular Med. 2003;4(1-2):37-48. doi: 10.1385/NMM:4:1-2:37. Neuromolecular Med. 2003. PMID: 14528051 Review. - The complexity of tau in Alzheimer's disease.
Naseri NN, Wang H, Guo J, Sharma M, Luo W. Naseri NN, et al. Neurosci Lett. 2019 Jul 13;705:183-194. doi: 10.1016/j.neulet.2019.04.022. Epub 2019 Apr 25. Neurosci Lett. 2019. PMID: 31028844 Free PMC article. Review.
References
- St George-Hyslop, P. H., Farrer, L. A. & Goedert, M. (2001) in The Metabolic and Molecular Bases of Inherited Disease, eds. Scriver, C. R., Beaudet, A. L., Sly, W. S. & Valle, D. (McGraw–Hill, New York), pp. 5875-5899.
- Crowther, R. A. & Goedert, M. (2000) J. Struct. Biol. 130, 271-279. - PubMed
- Goedert, M., Spillantini, M. G., Jakes, R., Rutherford, D. & Crowther, R. A. (1989) Neuron 3, 519-526. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources