A dual mechanism controlling the localization and function of exocytic v-SNAREs - PubMed (original) (raw)
. 2003 Jul 22;100(15):9011-6.
doi: 10.1073/pnas.1431910100. Epub 2003 Jul 9.
Rachel Rudge, Marcella Vacca, Graça Raposo, Jacques Camonis, Véronique Proux-Gillardeaux, Laurent Daviet, Etienne Formstecher, Alexandre Hamburger, Francesco Filippini, Maurizio D'Esposito, Thierry Galli
Affiliations
- PMID: 12853575
- PMCID: PMC166429
- DOI: 10.1073/pnas.1431910100
A dual mechanism controlling the localization and function of exocytic v-SNAREs
Sonia Martinez-Arca et al. Proc Natl Acad Sci U S A. 2003.
Abstract
SNARE [soluble NSF (N-ethylmaleimide-sensitive factor) attachment protein receptor] proteins are essential for membrane fusion but their regulation is not yet fully understood. We have previously shown that the amino-terminal Longin domain of the v-SNARE TI-VAMP (tetanus neurotoxin-insensitive vesicle-associated membrane protein)/VAMP7 plays an inhibitory role in neurite outgrowth. The goal of this study was to investigate the regulation of TI-VAMP as a model of v-SNARE regulation. We show here that the Longin domain (LD) plays a dual role. First, it negatively regulates the ability of TI-VAMP and of a Longin/Synaptobrevin chimera to participate in SNARE complexes. Second, it interacts with the adaptor complex AP-3 and this interaction targets TI-VAMP to late endosomes. Accordingly, in mocha cells lacking AP-3 delta, TI-VAMP is retained in an early endosomal compartment. Furthermore, TI-VAMPc, an isoform of TI-VAMP lacking part of the LD, does not interact with AP-3, and therefore is not targeted to late endosomes; however, this shorter LD still inhibits SNARE-complex formation. These findings support a mechanism controlling both localization and function of TI-VAMP through the LD and clathrin adaptors. Moreover, they point to the amino-terminal domains of SNARE proteins as multifunctional modules responsible for the fine tuning of SNARE function.
Figures
Fig. 1.
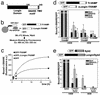
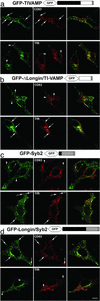
The LD inhibits SNARE-complex formation in vitro and in vivo. (a) Scheme of the structure of TI-VAMP. TMD, transmembrane domain. (b and c) Quantitative in vitro assay to measure the interaction between recombinant syntaxin 1 and SNAP25 with TI-VAMP or ΔLongin-TIVAMP. The graphic shows one representative experiment of three independent experiment. (d and e) HeLa cells transfected with GFP-TIVAMP or GFP-ΔLongin-TIVAMP (d) or with GFP-Syb2 or GFP-Longin/Syb2 (e), and treated as described in Fig. 6_c_, were lysed and immunoprecipitated with an anti-GFP antibody. Coimmunoprecipitated SNAREs were detected by Western blot and quantified by densitometry. The histograms show the statistical analyses of at least three independent experiments. *, P < 0.05, Student's t test.
Fig. 4.
Direct interaction between AP-3δ and the LD. (a) Results of a yeast two-hybrid screening using either the Longin or the cytoplasmic domain of Drosophila or human TI-VAMP as baits. SNAP-29, a yet unknown partner of TI-VAMP, was also detected both in the human and in the_Drosophila_ screening, and biochemical experiments validated this interaction (unpublished observations). The red and green lines highlight the intersection and total coverage of all prey clones identified in the yeast two-hybrid screen, respectively. (b) HeLa cells transfected with the GFP-fusion constructs indicated were crosslinked, lysed, and immunoprecipitated with an anti-GFP antibody. Coimmunoprecipitated proteins were detected by Western blot. (c) HeLa cells fixed and double stained for endogenous TI-VAMP and AP-1, AP-2, and AP-3 are shown. (Bar, 15 μm.)
Fig. 2.
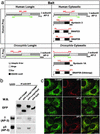
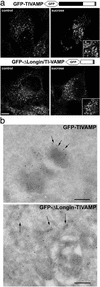
The LD controls the intracellular localization of TI-VAMP. (a) HeLa cells transfected with GFP-TIVAMP or GFP-ΔLongin/TIVAMP were either directly fixed (control) or fixed after sucrose treatment (sucrose). Note the swelling of GFP-TIVAMP-positive vesicles, but not that of GFP-ΔLongin-TIVAMP-positive vesicles (Insets). (Bar, 10 μm; bar in Insets,7 μm.) (b) Ultrathin cryosections of MDCK cells expressing GFP-TIVAMP and GFP-Δ Longin-TIVAMP were ImmunoGold-labeled with anti-GFP antibodies and PAG10. TI-VAMP localizes to the limiting membrane of lysosomal compartments (arrows). Occasionally, TI-VAMP is detected in small closely apposed vesicles (arrowheads). GFP-Δ Longin-TIVAMP localizes to tubular vesicular membrane structures (arrows). (Bars, 200 nm.)
Fig. 3.
The LD controls the intracellular localization of v-SNAREs. HeLa cells transfected with the constructs indicated were fixed and stained for the endosomal markers CD63 and TfR. Arrows point to structures labeled by both the GFP-fusion protein and either CD63 or TfR. Arrowheads point to structures positive for only one of the proteins. (Bar, 13 μm.)
Fig. 5.
Localization and function of TI-VAMP depends on its interaction with functional AP-3 complex. (a) Mocha cells were transfected with either GFP-TIVAMP or GFP-ΔLongin-TIVAMP alone, or were cotransfected with AP-3δ. GFP-TIVAMP and GFP-ΔLongin-TIVAMP were detected by direct GFP fluorescence (green), whereas AP-3δ (blue) and TfR (red) were detected by coimmunolabeling. Merge, the level of colocalization between the TfR and GFP-TIVAMP or GFP-ΔLongin-TIVAMP. (b) Scheme of the structure of TI-VAMP compared with TI-VAMPc. Isoform c lacks residues 28–68 (red box). (c) HeLa cells transfected with GFP-TIVAMPc were fixed and stained for endogenous CD63. (d) HeLa cells transfected with the GFP-fusion constructs indicated were crosslinked, lysed, and immunoprecipitated with an anti-GFP antibody. Coimmunoprecipitated proteins were detected by Western blot. (e) HeLa cells transfected with the GFP-fusion constructs indicated were incubated with NEM as in Fig. 6_c_, lysed, and immunoprecipitated with a mouse monoclonal anti-GFP antibody. Coimmunoprecipitated SNAREs were detected by Western blot. (Bar in a, 10 μm; bar in c, 13 μm.)
Similar articles
- Multiple roles of the vesicular-SNARE TI-VAMP in post-Golgi and endosomal trafficking.
Chaineau M, Danglot L, Galli T. Chaineau M, et al. FEBS Lett. 2009 Dec 3;583(23):3817-26. doi: 10.1016/j.febslet.2009.10.026. Epub 2009 Oct 20. FEBS Lett. 2009. PMID: 19837067 Review. - Expression of the Longin domain of TI-VAMP impairs lysosomal secretion and epithelial cell migration.
Proux-Gillardeaux V, Raposo G, Irinopoulou T, Galli T. Proux-Gillardeaux V, et al. Biol Cell. 2007 May;99(5):261-71. doi: 10.1042/BC20060097. Biol Cell. 2007. PMID: 17288539 - Role of tetanus neurotoxin insensitive vesicle-associated membrane protein (TI-VAMP) in vesicular transport mediating neurite outgrowth.
Martinez-Arca S, Alberts P, Zahraoui A, Louvard D, Galli T. Martinez-Arca S, et al. J Cell Biol. 2000 May 15;149(4):889-900. doi: 10.1083/jcb.149.4.889. J Cell Biol. 2000. PMID: 10811829 Free PMC article. - The tetanus neurotoxin-sensitive and insensitive routes to and from the plasma membrane: fast and slow pathways?
Proux-Gillardeaux V, Rudge R, Galli T. Proux-Gillardeaux V, et al. Traffic. 2005 May;6(5):366-73. doi: 10.1111/j.1600-0854.2005.00288.x. Traffic. 2005. PMID: 15813747 Review. - A novel tetanus neurotoxin-insensitive vesicle-associated membrane protein in SNARE complexes of the apical plasma membrane of epithelial cells.
Galli T, Zahraoui A, Vaidyanathan VV, Raposo G, Tian JM, Karin M, Niemann H, Louvard D. Galli T, et al. Mol Biol Cell. 1998 Jun;9(6):1437-48. doi: 10.1091/mbc.9.6.1437. Mol Biol Cell. 1998. PMID: 9614185 Free PMC article.
Cited by
- The longin SNARE VAMP7/TI-VAMP adopts a closed conformation.
Vivona S, Liu CW, Strop P, Rossi V, Filippini F, Brunger AT. Vivona S, et al. J Biol Chem. 2010 Jun 4;285(23):17965-73. doi: 10.1074/jbc.M110.120972. Epub 2010 Apr 8. J Biol Chem. 2010. PMID: 20378544 Free PMC article. - Cytotoxic Granule Exocytosis From Human Cytotoxic T Lymphocytes Is Mediated by VAMP7.
Chitirala P, Ravichandran K, Galgano D, Sleiman M, Krause E, Bryceson YT, Rettig J. Chitirala P, et al. Front Immunol. 2019 Aug 7;10:1855. doi: 10.3389/fimmu.2019.01855. eCollection 2019. Front Immunol. 2019. PMID: 31447853 Free PMC article. - v-SNARE cellubrevin is required for basolateral sorting of AP-1B-dependent cargo in polarized epithelial cells.
Fields IC, Shteyn E, Pypaert M, Proux-Gillardeaux V, Kang RS, Galli T, Fölsch H. Fields IC, et al. J Cell Biol. 2007 May 7;177(3):477-88. doi: 10.1083/jcb.200610047. J Cell Biol. 2007. PMID: 17485489 Free PMC article. - Effects of the SNAP-25 Mnll variant on hippocampal functional connectivity in children with attention deficit/hyperactivity disorder.
Huang W, Fateh AA, Zhao Y, Zeng H, Yang B, Fang D, Zhang L, Meng X, Hassan M, Wen F. Huang W, et al. Front Hum Neurosci. 2023 Aug 10;17:1219189. doi: 10.3389/fnhum.2023.1219189. eCollection 2023. Front Hum Neurosci. 2023. PMID: 37635807 Free PMC article. - VARP is recruited on to endosomes by direct interaction with retromer, where together they function in export to the cell surface.
Hesketh GG, Pérez-Dorado I, Jackson LP, Wartosch L, Schäfer IB, Gray SR, McCoy AJ, Zeldin OB, Garman EF, Harbour ME, Evans PR, Seaman MNJ, Luzio JP, Owen DJ. Hesketh GG, et al. Dev Cell. 2014 Jun 9;29(5):591-606. doi: 10.1016/j.devcel.2014.04.010. Epub 2014 May 22. Dev Cell. 2014. PMID: 24856514 Free PMC article.
References
- Rothman, J. E. (2002) Nat. Med. 8, 1059-1062. - PubMed
- Galli, T. & Haucke, V. (2001) Sci. STKE 2001, RE1. - PubMed
- Jahn, R., Lang, T. & Sudhof, T. C. (2003) Cell 112, 519-533. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials