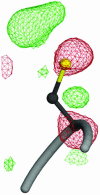The 1.1-A resolution crystal structure of DJ-1, the protein mutated in autosomal recessive early onset Parkinson's disease - PubMed (original) (raw)
The 1.1-A resolution crystal structure of DJ-1, the protein mutated in autosomal recessive early onset Parkinson's disease
Mark A Wilson et al. Proc Natl Acad Sci U S A. 2003.
Abstract
Mutations in DJ-1, a human gene with homologues in organisms from all kingdoms of life, have been shown to be associated with autosomal recessive, early onset Parkinson's disease (PARK7). We report here the three-dimensional structure of the DJ-1 protein, determined at a resolution of 1.1 A by x-ray crystallography. The chain fold of DJ-1 resembles those of a bacterial protein, PfpI, that has been annotated as a cysteine protease, and of a domain of a bacterial catalase whose role in the activity of that enzyme is uncertain. In contrast to PfpI, a hexameric protein whose oligomeric structure is essential for its putative proteolytic activity, DJ-1 is a dimer with completely different intersubunit contacts. The proposed catalytic triad of PfpI is absent from the corresponding region of the structure of DJ-1, and biochemical assays fail to detect any protease activity for purified DJ-1. A highly conserved cysteine residue, which is catalytically essential in homologues of DJ-1, shows an extreme sensitivity to radiation damage and may be subject to other forms of oxidative modification as well. The structure suggests that the loss of function caused by the Parkinson's-associated mutation L166P in DJ-1 is due to destabilization of the dimer interface. Taken together, the crystal structure of human DJ-1 plus other observations suggest the possible involvement of this protein in the cellular oxidative stress response and a general etiology of neurodegenerative diseases.
Figures
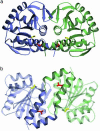
Fig. 1.
(a and b) Two views of the DJ-1 crystallographic dimer. Monomer A is purple and monomer B is green. The view in b is rotated by 90° with respect to the view in a. In both views, Cys-106 is yellow and Leu-166, which is mutated to proline in PARK7 familial PD, is red. In b, the unusual coaxial arrangement of the two C-terminal α-helices (G and H) at the dimer interface is highlighted. The figure was made with
povscript+
(50).
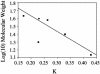
Fig. 2.
Gel filtration chromatography indicates that DJ-1 is a dimer in solution. The observed elution of DJ-1 is plotted against the expected molecular mass of the monomer (•) and the dimer (▾). The dimeric molecular weight agrees well with the best-fit line for the calibration standards (▪), whereas the monomer does not.
Fig. 3.
A view of radiation damage around Cys-106 at the nucleophile elbow region. Fourier difference (_F_o – _F_c) electron density is shown contoured at +3.0 σ (green) and –3.0 σ (red). The pronounced difference electron density around this residue indicates that it is particularly sensitive to radiation-induced structural changes and possibly prone to other oxidative modification. The figure was made with POVSCRIPT+ (50).
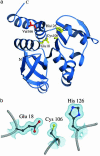
Fig. 4.
Residues near the nucleophile elbow region of DJ-1 do not comprise a catalytic triad. (a) A ribbon diagram of the DJ-1 monomer is shown, with residues Glu-18, Cys-106, and His-126 in yellow. Leu-166, which is mutated to proline in PARK7 familial PD, is red. (b) A closer view of the nucleophile elbow region with 2_F_o –_F_c electron density contoured at 1.0 σ. Although the identity of the residues is correct for a catalytic triad, their conformation prohibits proton shuttling by the canonical mechanism of a serine/cysteine protease or a glutamine amidotransferase. The figure was made with
povscript+
(50).
Comment in
- Crystallizing ideas about Parkinson's disease.
Cookson MR. Cookson MR. Proc Natl Acad Sci U S A. 2003 Aug 5;100(16):9111-3. doi: 10.1073/pnas.1633722100. Epub 2003 Jul 28. Proc Natl Acad Sci U S A. 2003. PMID: 12886009 Free PMC article. No abstract available.
Similar articles
- Crystal structure of human DJ-1, a protein associated with early onset Parkinson's disease.
Tao X, Tong L. Tao X, et al. J Biol Chem. 2003 Aug 15;278(33):31372-9. doi: 10.1074/jbc.M304221200. Epub 2003 May 21. J Biol Chem. 2003. PMID: 12761214 - Crystal structure of DJ-1/RS and implication on familial Parkinson's disease.
Huai Q, Sun Y, Wang H, Chin LS, Li L, Robinson H, Ke H. Huai Q, et al. FEBS Lett. 2003 Aug 14;549(1-3):171-5. doi: 10.1016/s0014-5793(03)00764-6. FEBS Lett. 2003. PMID: 12914946 - Differential effects of Parkinson's disease-associated mutations on stability and folding of DJ-1.
Görner K, Holtorf E, Odoy S, Nuscher B, Yamamoto A, Regula JT, Beyer K, Haass C, Kahle PJ. Görner K, et al. J Biol Chem. 2004 Feb 20;279(8):6943-51. doi: 10.1074/jbc.M309204200. Epub 2003 Nov 7. J Biol Chem. 2004. PMID: 14607841 - DJ-1 and prevention of oxidative stress in Parkinson's disease and other age-related disorders.
Kahle PJ, Waak J, Gasser T. Kahle PJ, et al. Free Radic Biol Med. 2009 Nov 15;47(10):1354-61. doi: 10.1016/j.freeradbiomed.2009.08.003. Epub 2009 Aug 14. Free Radic Biol Med. 2009. PMID: 19686841 Review. - Linking DJ-1 to neurodegeneration offers novel insights for understanding the pathogenesis of Parkinson's disease.
Bonifati V, Oostra BA, Heutink P. Bonifati V, et al. J Mol Med (Berl). 2004 Mar;82(3):163-74. doi: 10.1007/s00109-003-0512-1. Epub 2004 Jan 8. J Mol Med (Berl). 2004. PMID: 14712351 Review.
Cited by
- Effect of single amino acid substitution on oxidative modifications of the Parkinson's disease-related protein, DJ-1.
Madian AG, Hindupur J, Hulleman JD, Diaz-Maldonado N, Mishra VR, Guigard E, Kay CM, Rochet JC, Regnier FE. Madian AG, et al. Mol Cell Proteomics. 2012 Feb;11(2):M111.010892. doi: 10.1074/mcp.M111.010892. Epub 2011 Nov 21. Mol Cell Proteomics. 2012. PMID: 22104028 Free PMC article. - DJ-1 deficient mice demonstrate similar vulnerability to pathogenic Ala53Thr human alpha-syn toxicity.
Ramsey CP, Tsika E, Ischiropoulos H, Giasson BI. Ramsey CP, et al. Hum Mol Genet. 2010 Apr 15;19(8):1425-37. doi: 10.1093/hmg/ddq017. Epub 2010 Jan 20. Hum Mol Genet. 2010. PMID: 20089532 Free PMC article. - Polymerization of Oxidized DJ-1 via Noncovalent and Covalent Binding: Significance of Disulfide Bond Formation.
Kobayashi M, Muramatsu K, Haruyama T, Uesugi H, Kikuchi A, Konno H, Noguchi N, Saito Y. Kobayashi M, et al. ACS Omega. 2019 Jun 3;4(6):9603-9614. doi: 10.1021/acsomega.9b00324. eCollection 2019 Jun 30. ACS Omega. 2019. PMID: 31460051 Free PMC article. - Shining light on cysteine modification: connecting protein conformational dynamics to catalysis and regulation.
van den Bedem H, Wilson MA. van den Bedem H, et al. J Synchrotron Radiat. 2019 Jul 1;26(Pt 4):958-966. doi: 10.1107/S160057751900568X. Epub 2019 Jun 13. J Synchrotron Radiat. 2019. PMID: 31274417 Free PMC article. - Crystal structure of filamentous aggregates of human DJ-1 formed in an inorganic phosphate-dependent manner.
Cha SS, Jung HI, Jeon H, An YJ, Kim IK, Yun S, Ahn HJ, Chung KC, Lee SH, Suh PG, Kang SO. Cha SS, et al. J Biol Chem. 2008 Dec 5;283(49):34069-75. doi: 10.1074/jbc.M804243200. Epub 2008 Oct 14. J Biol Chem. 2008. PMID: 18922803 Free PMC article.
References
- Olanow, C. W. & Tatton, W. G. (1999) Annu. Rev. Neurosci. 22, 123–144. - PubMed
- Parkinson, J. (1817) An Essay on the Shaking Palsy (Sherwood, Neely, and Jones, London).
- Langston, J. W., Ballard, P., Tetrud, J. W. & Irwin, I. (1983) Science 219, 979–980. - PubMed
- Gershanik, O. S. (2002) in Parkinson's Disease and Movement Disorders, eds. Jankovic, J. J. & Tolosa, E. (Lippincott, Williams & Wilkins, Baltimore), pp. 331–357.
- Tretiakoff, C. (1919) Thèse de Médicine (University of Paris, Paris).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous