The degradation of promyelocytic leukemia and Sp100 proteins by herpes simplex virus 1 is mediated by the ubiquitin-conjugating enzyme UbcH5a - PubMed (original) (raw)
The degradation of promyelocytic leukemia and Sp100 proteins by herpes simplex virus 1 is mediated by the ubiquitin-conjugating enzyme UbcH5a
Haidong Gu et al. Proc Natl Acad Sci U S A. 2003.
Abstract
Infected cell protein 0 (ICP0) of herpes simplex virus 1 expresses two E3 ubiquitin (Ub) ligase activities mapping in the domains encoded by exons 2 and 3, respectively. Site 1 (exon 3) is responsible for the degradation of the E2 Ub-conjugating enzyme cdc34 whereas site 2 (exon 2) is associated with a ring finger and has been shown to mediate the degradation of promyelocytic leukemia (PML) and Sp100 proteins and the dispersal of nuclear domain 10 (ND10). In in vitro assays site 2 polyubiquitylates the E2 enzymes UbcH5a and UbcH6 but not other (e.g., UbcH7) enzymes. In this article, we show that ectopic expression of dominant negative UbcH5a carrying the substitution C85A delayed or blocked the degradation of PML and Sp100 and dispersal of ND10 whereas ectopic expression of wild-type UbcH5a or dominant negative UbcH6 and UbcH7 carrying the substitutions C131A and C86A, respectively, had no effect. These results link the degradation of PML and Sp100 and the dispersal of ND10 to the E3 activities of ICP0 associated with the UbcH5a E2 enzyme.
Figures
Fig. 1.
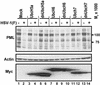
Expression of ICP0 and accumulation of PML in infected cells. (A) Accumulation of PML and ICP0 in three cells lines exposed to ratios of PFU per cell shown, incubated for 24 h after virus exposure, and processed as described in Materials and Methods. The 6% denaturing polyacrylamide gels were loaded with 40 μg of total protein per lane. The HSV-1(F) stock was titered in Vero cells. Mock, mock-infected cells; cells, cells maintained in growth medium and neither mock-infected nor infected. The electrophoretically separated proteins were reacted with antibodies as described in Materials and Methods. (B) PML degradation in SK-N-SH cells. Zero time is 1 h after initial exposure of cells to virus. Cells were harvested at 2 and 12 h after mock infection and at times shown after HSV-1(F) infection (0.5 PFU per cell). The harvested cells were processed as described in Materials and Methods and in the legend to_A_.
Fig. 2.
Accumulation of PML in SK-N-SH cells mock-infected or infected with HSV-1(F) 24 h after they were mock-transduced with insect cell medium or transduced with baculoviruses expressing wt or dn UbcH5a, UbcH6, or UbcH7. The multiplicities of infection were 10 PFU of baculoviruses and 5 PFU of HSV-1(F) per cell. To enhance the expression of genes encoded in baculovirus vectors, the cultures were incubated in medium containing 5 mM sodium butyrate after transduction. The cells were harvested 30 min after the 1-h exposure to HSV-1(F), electrophoretically separated in a denaturing 4–20% gradient polyacrylamide gel, and reacted with antibodies as described in Materials and Methods. Actin served as a gel loading control.
Fig. 3.
Accumulation of PML and Sp100 protein in cells transduced with dnUbcH5a. (A) Accumulation of PML in SK-N-SH cells transduced with various ratios of recombinant baculoviruses encoding dnUbcH5a per cell. The experiment was carried out as described in the legend to Fig. 2 except that the multiplicity of infection with baculovirus encoding dnUbcH5a was varied as shown. (B) Effect of the length of incubation of transduced cells on the accumulation of PML in SK-N-SH cells infected with HSV-1(F). The cells were transduced with 10 PFU of baculovirus encoding dnUbcH5a per cell. At the times shown the cells were infected with HSV-1(F). Lanes 1 and 2, the cells were mock-transduced by exposure to insect cell medium. (C) Effect of the time of gene expression of the transducing recombinant baculoviruses on the accumulation of Sp100 in SK-N-SH cells infected with HSV-1(F). The experiment was done as described in the legend to B except that the cells were treated with 1,000 units of IFN-γ per ml concurrent with 5 mM sodium butyrate to enable the visualization of Sp100 protein and reacted with antibodies as described in Materials and Methods.
Fig. 4.
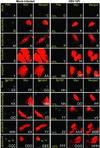
Accumulation of PML (A_–_DD) or Sp100 proteins (EE_–_HHH) in SK-N-SH cells mock-transduced with insect cell medium or baculoviruses encoding wtUbcH5a or dnUbcH5a, dnUbcH6, or dnUbcH7. The cells were transduced with baculoviruses and incubated in medium containing sodium butyrate (A_–_DD) or both sodium butyrate and 1,000 units of IFN-γ per ml (EE_–_HHH). The cells were fixed 4 h after infection with HSV-1(F) and reacted with anti-PML or anti-Sp100 protein antibody (FITC) or anti-myc tag antibody (Texas red) as described in Materials and Methods. The left three columns are representative of mock-infected cells. The right three columns are representative of the HSV-1(F)-infected cells. The numbers in the fourth column indicate the percent of cells that contained PML or Sp100 proteins in discernible ND10 structures and that were either mock-transduced (A_–_F and_EE_–JJ) or transduced with baculoviruses encoding wt or dn E2 enzymes (G_–_DD or_KK_–HHH). The percentages are based on counts of ≈200 cells with the aid of high magnification (×63 objective) in a Zeiss confocal microscope.
Fig. 5.
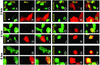
Distribution of ICP0 in SK-N-SH cells exposed to insect cell medium or baculoviruses encoding wtUbcH5a, dnUbcH5a, or dnUbcH6 and infected with HSV-1(F). The procedure for the treatment of the slide cultures was the same as described in Fig. 4 except that the cells were fixed at 2, 4, or 8 h after HSV-1(F) infection and reacted with anti-ICP0 (FITC) and anti-myc (Texas red) antibodies. The arrows point to cells containing ICP0 in speckled nuclear structures. In other assays these speckled structures were juxtaposed to or overlapped ND10 structures (data not shown).
Similar articles
- Herpes simplex virus 1-infected cell protein 0 contains two E3 ubiquitin ligase sites specific for different E2 ubiquitin-conjugating enzymes.
Hagglund R, Van Sant C, Lopez P, Roizman B. Hagglund R, et al. Proc Natl Acad Sci U S A. 2002 Jan 22;99(2):631-6. doi: 10.1073/pnas.022531599. Proc Natl Acad Sci U S A. 2002. PMID: 11805320 Free PMC article. - Two overlapping regions within the N-terminal half of the herpes simplex virus 1 E3 ubiquitin ligase ICP0 facilitate the degradation and dissociation of PML and dissociation of Sp100 from ND10.
Perusina Lanfranca M, Mostafa HH, Davido DJ. Perusina Lanfranca M, et al. J Virol. 2013 Dec;87(24):13287-96. doi: 10.1128/JVI.02304-13. Epub 2013 Oct 2. J Virol. 2013. PMID: 24089549 Free PMC article. - Nuclear dots: actors on many stages.
Sternsdorf T, Grötzinger T, Jensen K, Will H. Sternsdorf T, et al. Immunobiology. 1997 Dec;198(1-3):307-31. doi: 10.1016/S0171-2985(97)80051-4. Immunobiology. 1997. PMID: 9442402 Review. - PML and COP1--two proteins with much in common.
Reyes JC. Reyes JC. Trends Biochem Sci. 2001 Jan;26(1):18-20. doi: 10.1016/s0968-0004(00)01732-1. Trends Biochem Sci. 2001. PMID: 11165511 Review.
Cited by
- During its nuclear phase the multifunctional regulatory protein ICP0 undergoes proteolytic cleavage characteristic of polyproteins.
Gu H, Poon AP, Roizman B. Gu H, et al. Proc Natl Acad Sci U S A. 2009 Nov 10;106(45):19132-7. doi: 10.1073/pnas.0910920106. Epub 2009 Oct 22. Proc Natl Acad Sci U S A. 2009. PMID: 19850872 Free PMC article. - A viral E3 ligase targets RNF8 and RNF168 to control histone ubiquitination and DNA damage responses.
Lilley CE, Chaurushiya MS, Boutell C, Landry S, Suh J, Panier S, Everett RD, Stewart GS, Durocher D, Weitzman MD. Lilley CE, et al. EMBO J. 2010 Mar 3;29(5):943-55. doi: 10.1038/emboj.2009.400. Epub 2010 Jan 14. EMBO J. 2010. PMID: 20075863 Free PMC article. - UBE2Q1, as a Down Regulated Gene in Pediatric Acute Lymphoblastic Leukemia.
Seghatoleslam A, Bozorg-Ghalati F, Monabati A, Nikseresht M, Owji AA. Seghatoleslam A, et al. Int J Mol Cell Med. 2014 Spring;3(2):95-101. Int J Mol Cell Med. 2014. PMID: 25035859 Free PMC article. - Immediate-Early (IE) gene regulation of cytomegalovirus: IE1- and pp71-mediated viral strategies against cellular defenses.
Torres L, Tang Q. Torres L, et al. Virol Sin. 2014 Dec;29(6):343-52. doi: 10.1007/s12250-014-3532-9. Epub 2014 Nov 25. Virol Sin. 2014. PMID: 25501994 Free PMC article. Review.
References
- Roizman, B. & Knipe, D. M. (2001) in Fields Virology, eds. Knipe, D. M., Howley, P., Griffin, D. E., Lamb, R. A. Martin, M. A., Roizman, B. & Straus, S. E. (Lippincott-Williams and Wilkins, New York), 4th Ed., pp. 2399-2459.
Publication types
MeSH terms
Substances
Grants and funding
- CA78766/CA/NCI NIH HHS/United States
- CA83939/CA/NCI NIH HHS/United States
- R01 CA078766/CA/NCI NIH HHS/United States
- CA71933/CA/NCI NIH HHS/United States
- CA87661/CA/NCI NIH HHS/United States
- P01 CA087661/CA/NCI NIH HHS/United States
- P01 CA071933/CA/NCI NIH HHS/United States
- R01 CA088860/CA/NCI NIH HHS/United States
- CA88860/CA/NCI NIH HHS/United States
- R37 CA078766/CA/NCI NIH HHS/United States
- R01 CA083939/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials