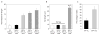Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts - PubMed (original) (raw)
Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts
Simona Parrinello et al. Nat Cell Biol. 2003 Aug.
Erratum in
- Nat Cell Biol. 2003 Sep;5(9):839
Abstract
Most mammalian cells do not divide indefinitely, owing to a process termed replicative senescence. In human cells, replicative senescence is caused by telomere shortening, but murine cells senesce despite having long stable telomeres. Here, we show that the phenotypes of senescent human fibroblasts and mouse embryonic fibroblasts (MEFs) differ under standard culture conditions, which include 20% oxygen. MEFs did not senesce in physiological (3%) oxygen levels, but underwent a spontaneous event that allowed indefinite proliferation in 20% oxygen. The proliferation and cytogenetic profiles of DNA repair-deficient MEFs suggested that DNA damage limits MEF proliferation in 20% oxygen. Indeed, MEFs accumulated more DNA damage in 20% oxygen than 3% oxygen, and more damage than human fibroblasts in 20% oxygen. Our results identify oxygen sensitivity as a critical difference between mouse and human cells, explaining their proliferative differences in culture, and possibly their different rates of cancer and ageing.
Conflict of interest statement
COMPETING FINANCIAL INTERESTS
The authors declare that they have no competing financial interests.
Figures
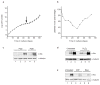
Figure 1
Senescent MEFs resemble oxidant-treated human fibroblasts. (a) Replicative lifespan, senescence (arrow) and spontaneous immortalization (resumed increase in cell number) is shown for the average of three independent C57Bl/6 MEF cultures (±s.d. indicated by error bars). (b) The percentage of labelled nuclei for a C57Bl/6 MEF culture were calculated at each passage for 55 days. Three independent cultures gave similar results. (c) Either early passage (PD2) or senescent (PD9) C57Bl/6 MEFs were analysed by western blotting for c-Fos and α-tubulin (control). Proteins (30 μg) from proliferating (lane 1), serum-deprived (lanes 2, 4) or serum-stimulated (lanes 3, 5) were analysed. (d) 82-6 human fibroblasts were treated with 400 μM hydrogen peroxide for 2 h. After 7 days, control and treated cells were maintained in 10% FCS (lanes 1 and 4), serum-deprived (lanes 2 and 5) or serum-deprived and stimulated (lanes 3 and 6). Proteins were analysed by western blotting for c-Fos and α-tubulin. c-Fos induction was detected 7 and 14 days after treatment with 200, 400 or 550 μM hydrogen peroxide. (e) Human fibroblasts were induced to senesce by infection with pLXSN-p14ARF (ref. 13) or treatment with 20 μg ml−1 bleomycin for 2 h (ref.12). After 7 days, control (untreated), infected (ARF) and treated (Bleo) cells were serum-deprived (lanes 1, 3 and 5) or serum-stimulated (lanes 2, 4 and 6) and analysed by western blotting for c-Fos and α-tubulin.
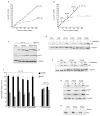
Figure 2
Low oxygen abolishes replicative senescence of MEFs. C57Bl/6 MEFs were used for all experiments. (a) MEFs were cultured in 20% or 3% oxygen, as indicated, and cell number was determined at each passage. The average and standard deviations of three independent cultures are shown. (b) MEFs cultured in 3% oxygen (black) were shifted at PD8, PD13 or PD19 to 20% oxygen (grey), or maintained in 3% oxygen. Cell number was determined at the indicated times. The average of two cultures is shown. (c) MEFs cultured in 3% or 20% oxygen were assayed for levels of p16, p19ARF and α-tubulin (control) by western blotting. 3% oxygen cultures were analysed at early (PD2) and late (PD14 and PD24) passage. A senescent 20% oxygen culture (PD9) is shown for comparison. (d) Six 3% oxygen MEF cultures at the indicated population doublings were analysed for levels of p53 and α-tubulin by western blotting before (−) or 1 h after X irradiation (+; 4.5 Gy). (e) An early passage (PD5) and two late-passage (PD36 and PD38) 3% oxygen MEF cultures were analysed for levels of p21 and α-tubulin before (−), 6 h and 14 h after X irradiation (4.5 Gy). (f) Six 3% oxygen MEF cultures at the indicated population doublings and two 20% oxygen immortal cultures were infected with control (black bars) or p19ARF-expressing (grey bars) retroviruses. After 48 h, 3H-thymidine was added for 1 h and the S-phase fraction determined (percentage labelled nuclei). (g) An early passage (PD5) and two late-passage (PD36 and PD38) 3% oxygen MEF cultures were infected with insertless vector (V) or Ha-RasV12-expressing (R) retroviruses and assayed for p19ARF, p53, Ras and α-tubulin by western blotting. (h) Exponentially growing (+) and serum-deprived (−) early passage (PD5) and late-passage (PD36 and PD38) 3% oxygen MEFs were assayed for levels of Rb by western blotting. Equal loading was confirmed by Ponceau S staining.
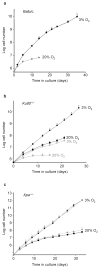
Figure 3
Growth of repair-deficient MEFs in 3% and 20% oxygen. (a) The replicative lifespans of three independent Balb/c MEF cultures maintained in 3% oxygen and two cultures maintained in 20% oxygen are shown. Error bars indicate the standard deviations. (b) Replicative lifespans and standard deviations of five Ku80−/− (grey) and three wild-type littermate (black) MEF cultures in 3% (circles) and 20% (squares) oxygen were determined. (c) The replicative lifespans and standard deviations of two Xpa−/− (grey) and two wild-type littermate (black) MEFs cultures grown in 3% (circles) and 20% (squares) oxygen are shown.
Figure 4
MEFs accumulate high levels of oxidative DNA damage in 20% oxygen. (a) The Fpg-comet assay was performed on early passage C57Bl/6 MEFs cultured in 3% or 20% oxygen, immortal MEFs cultures derived and grown in 20% oxygen and mid-lifespan (PD 35) WI-38 human fibroblasts cultured for more than 10 population doublings in 20% oxygen. The normalized average tail length is plotted. Each value represents the average of four independent experiments and at least 50 cells per determination. (b) Comet assay controls. WI38 and MEFs grown at the indicated oxygen concentrations and at the indicated population doublings were analysed for comet tail lengths before treatment with fpg. As a positive control, the normalized tail length of MEFs treated with hydrogen peroxide (100 μM) is also shown. (c) C57Bl/6 MEFs were derived and grown in 3% or 20% oxygen for up to 25 passages. The average percentage of metaphases from 4–5 independent cultures containing obvious chromosomal breaks or fragments is plotted. Error bars show s.e.m.
Similar articles
- DNA damage response activation in mouse embryonic fibroblasts undergoing replicative senescence and following spontaneous immortalization.
Di Micco R, Cicalese A, Fumagalli M, Dobreva M, Verrecchia A, Pelicci PG, di Fagagna Fd. Di Micco R, et al. Cell Cycle. 2008 Nov 15;7(22):3601-6. doi: 10.4161/cc.7.22.7152. Epub 2008 Nov 8. Cell Cycle. 2008. PMID: 19001874 - A human-like senescence-associated secretory phenotype is conserved in mouse cells dependent on physiological oxygen.
Coppé JP, Patil CK, Rodier F, Krtolica A, Beauséjour CM, Parrinello S, Hodgson JG, Chin K, Desprez PY, Campisi J. Coppé JP, et al. PLoS One. 2010 Feb 12;5(2):e9188. doi: 10.1371/journal.pone.0009188. PLoS One. 2010. PMID: 20169192 Free PMC article. - Down-Regulation of Fibroblast Growth Factor 2 (FGF2) Contributes to the Premature Senescence of Mouse Embryonic Fibroblast.
Li J, Song S, Li X, Zhu J, Li W, Du B, Guo Y, Xi X, Han R. Li J, et al. Med Sci Monit. 2020 Mar 19;26:e920520. doi: 10.12659/MSM.920520. Med Sci Monit. 2020. PMID: 32188838 Free PMC article. - Methods for cell sorting of young and senescent cells.
Passos JF, von Zglinicki T. Passos JF, et al. Methods Mol Biol. 2007;371:33-44. doi: 10.1007/978-1-59745-361-5_4. Methods Mol Biol. 2007. PMID: 17634572 Review. - Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes.
Toussaint O, Medrano EE, von Zglinicki T. Toussaint O, et al. Exp Gerontol. 2000 Oct;35(8):927-45. doi: 10.1016/s0531-5565(00)00180-7. Exp Gerontol. 2000. PMID: 11121681 Review.
Cited by
- Computational image analysis of nuclear morphology associated with various nuclear-specific aging disorders.
Choi S, Wang W, Ribeiro AJ, Kalinowski A, Gregg SQ, Opresko PL, Niedernhofer LJ, Rohde GK, Dahl KN. Choi S, et al. Nucleus. 2011 Nov-Dec;2(6):570-9. doi: 10.4161/nucl.2.6.17798. Epub 2011 Nov 1. Nucleus. 2011. PMID: 22127259 Free PMC article. - Interphase Chromosomes in Replicative Senescence: Chromosome Positioning as a Senescence Biomarker and the Lack of Nuclear Motor-Driven Chromosome Repositioning in Senescent Cells.
Mehta IS, Riyahi K, Pereira RT, Meaburn KJ, Figgitt M, Kill IR, Eskiw CH, Bridger JM. Mehta IS, et al. Front Cell Dev Biol. 2021 May 24;9:640200. doi: 10.3389/fcell.2021.640200. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34113611 Free PMC article. - Cellular senescence: a hitchhiker's guide.
Aravinthan A. Aravinthan A. Hum Cell. 2015 Apr;28(2):51-64. doi: 10.1007/s13577-015-0110-x. Epub 2015 Feb 18. Hum Cell. 2015. PMID: 25690721 Review. - Axonal Growth Arrests After an Increased Accumulation of Schwann Cells Expressing Senescence Markers and Stromal Cells in Acellular Nerve Allografts.
Poppler LH, Ee X, Schellhardt L, Hoben GM, Pan D, Hunter DA, Yan Y, Moore AM, Snyder-Warwick AK, Stewart SA, Mackinnon SE, Wood MD. Poppler LH, et al. Tissue Eng Part A. 2016 Jul;22(13-14):949-61. doi: 10.1089/ten.TEA.2016.0003. Epub 2016 Jul 7. Tissue Eng Part A. 2016. PMID: 27297909 Free PMC article. - SOD2 deficiency promotes aging phenotypes in mouse skin.
Weyemi U, Parekh PR, Redon CE, Bonner WM. Weyemi U, et al. Aging (Albany NY). 2012 Feb;4(2):116-8. doi: 10.18632/aging.100433. Aging (Albany NY). 2012. PMID: 22328603 Free PMC article. Review. No abstract available.
References
- Wright WE, Shay JW. Telomere dynamics in cancer progression and prevention: fundamental differences in human and mouse telomere biology. Nature Med. 2000;6:849–851. - PubMed
- Campisi J. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol. 2001;11:27–31. - PubMed
- Shelton DN, Chang E, Whittier PS, Choi D, Funk WD. Microarray analysis of replicative senescence. Curr Biol. 1999;9:939–945. - PubMed
- Serrano M, Blasco MA. Putting the stress on senescence. Curr Opin Cell Biol. 2001;13:748–753. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- Z01 BC010658/ImNIH/Intramural NIH HHS/United States
- T32 AG000266/AG/NIA NIH HHS/United States
- AG18679/AG/NIA NIH HHS/United States
- AG17242/AG/NIA NIH HHS/United States
- P01 AG017242/AG/NIA NIH HHS/United States
- R01 AG018679/AG/NIA NIH HHS/United States
- AG00266/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources