Reprogramming the host response in bacterial meningitis: how best to improve outcome? - PubMed (original) (raw)
Review
Reprogramming the host response in bacterial meningitis: how best to improve outcome?
M van der Flier et al. Clin Microbiol Rev. 2003 Jul.
Abstract
Despite effective antibiotic therapy, bacterial meningitis is still associated with high morbidity and mortality in both children and adults. Animal studies have shown that the host inflammatory response induced by bacterial products in the subarachnoid space is associated with central nervous system injury. Thus, attenuation of inflammation early in the disease process might improve the outcome. The feasibility of such an approach is demonstrated by the reduction in neurologic sequelae achieved with adjuvant dexamethasone therapy. Increased understanding of the pathways of inflammation and neuronal damage has suggested rational new targets to modulate the host response in bacterial meningitis, but prediction of which agents would be optimal has been difficult. This review compares the future promise of benefit from the use of diverse adjuvant agents. It appears unlikely that inhibition of a single proinflammatory mediator will prove useful in clinical practice, but several avenues to reprogram a wider array of mediators simultaneously are encouraging. Particularly promising are efforts to adjust combinations of cytokines, to inhibit neuronal apoptosis and to enhance brain repair.
Figures
FIG. 1.
Targeting of bacterial cell wall, cell membrane components, and toxins to prevent injury. Use of nonlytic antibiotic agents may minimize the release of toxic components following initiation of antibiotic therapy. Different organisms and different bacterial substances require separate neutralizing antagonists, making a uniform strategy difficult to design.
FIG. 2.
Interventions aimed at cytokines may target different levels: regulation of cytokine production via interference with transcriptin factors, interference with procytokine activation by proteolytic enzyme inhibition, direct blocking of the cytokine, and interference with the cytokine receptor or signaling pathway.
FIG. 3.
Interference with inflammatory pathways may target a whole spectrum of noncytokine mediators. Several mediators influence both the inflammatory and coagulation cascades. NSAID's. nonsteroidal anti-inflammatory drugs. ATIII, antithrombin III.
FIG. 4.
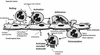
Process of neutrophil transendothelial migration. Initially the neutrophil is slowed and rolls down the endothelium, following thethering by an interaction of endothelial E-selectin and P-selectin with the leukocyte CD15 sialyl Lewis-X carbohydrate moieties and an interaction of L-selectin with endothelial cell carbohydrate moieties. Chemokine binding to receptors on leukocytes activates leukocyte adhesins of the integrin family, such as the β2αM integrin CD18/CD11b. Simultaneously, mediators such as IL-8 upregulate the endothelial adhesins of the immunoglobulin superfamily (e.g., intercellular adhesin molecule 1 [ICAM-1]) which bind CD18 on the leukocyte, initiating diapedesis into the cerebrospinal fluid. Matrix metalloproteinases released by the leukocyte digest intercellular tight junctions and the basal membrane during diapedesis.
FIG. 5.
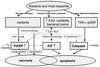
Neuronal cell death pathways may be divided into necrotic pathways, caspase-independent apoptotic pathway, and caspase-dependent apoptotic pathway. Bacterial and host oxidants cause damage to cell membranes via lipid peroxidation, leading to loss of membrane integrity and depolarization and finally necrotic cell death. Oxidants also cause DNA damage, resulting in the energy-consuming activation of the poly(ADP-ribose) polymerase (PARP). When DNA repair is futile because of the magnitude of the damage, massive energy depletion will cause necrotic cell death. Oxidants, high concentrations of excitatory amino acids (EAA), and bacterial toxins such as the pneumolysin of S. pneumoniae or the hemolysin of S. agalactiae all cause increased cytosolic free calcium levels. This may result in PARP activation and contribute to necrotic death but primarily causes damage to the mitochondrial outermembranes with PARP-dependent release of apoptosis-inducing factor (AIF). Free cytosolic apoptosis-inducing factor will move into the nucleus and cause chromatin condensation and apoptotic cell death. Release of inflammatory mediators such as TNF-α in response to the invading bacterial pathogens will result in activation of caspases. This results in activation of the apoptotic pathway and will also inhibit necrosis through inactivation of PARP. Release of cytochrome c from mitochondria following mitochondrial damage in response to increased cytosolic free calcium levels also causes activation of caspases and apoptotic death. As indicated in the figure, several crossroads exist between the different death pathways. In many cases, both necrotic and apoptotic cell death may be revealed on histologic examination.
Similar articles
- Mechanisms of brain injury in bacterial meningitis: workshop summary.
Pfister HW, Fontana A, Täuber MG, Tomasz A, Scheld WM. Pfister HW, et al. Clin Infect Dis. 1994 Sep;19(3):463-79. doi: 10.1093/clinids/19.3.463. Clin Infect Dis. 1994. PMID: 7811866 - [Current views on the pathophysiology of bacterial meningitis--new therapeutic possibilities?].
Kepa L. Kepa L. Pol Merkur Lekarski. 1997 Dec;3(18):291-4. Pol Merkur Lekarski. 1997. PMID: 9523471 Review. Polish. - Pathogenesis of bacterial meningitis.
Leib SL, Täuber MG. Leib SL, et al. Infect Dis Clin North Am. 1999 Sep;13(3):527-48, v-vi. doi: 10.1016/s0891-5520(05)70093-3. Infect Dis Clin North Am. 1999. PMID: 10470554 Review. - New understandings on the pathophysiology of bacterial meningitis.
Koedel U, Klein M, Pfister HW. Koedel U, et al. Curr Opin Infect Dis. 2010 Jun;23(3):217-23. doi: 10.1097/QCO.0b013e328337f49e. Curr Opin Infect Dis. 2010. PMID: 20216309 Review. - Pathophysiology of bacterial meningitis: mechanism(s) of neuronal injury.
Scheld WM, Koedel U, Nathan B, Pfister HW. Scheld WM, et al. J Infect Dis. 2002 Dec 1;186 Suppl 2:S225-33. doi: 10.1086/344939. J Infect Dis. 2002. PMID: 12424702 Review.
Cited by
- Death is associated with complement C3 depletion in cerebrospinal fluid of patients with pneumococcal meningitis.
Goonetilleke UR, Scarborough M, Ward SA, Hussain S, Kadioglu A, Gordon SB. Goonetilleke UR, et al. mBio. 2012 Mar 13;3(2):e00272-11. doi: 10.1128/mBio.00272-11. Print 2012. mBio. 2012. PMID: 22415003 Free PMC article. - Combined therapy with ceftriaxone and doxycycline does not improve the outcome of meningococcal meningitis in mice compared to ceftriaxone monotherapy.
Ricci S, Grandgirard D, Masouris I, Braccini T, Pozzi G, Oggioni MR, Koedel U, Leib SL. Ricci S, et al. BMC Infect Dis. 2020 Jul 13;20(1):505. doi: 10.1186/s12879-020-05226-w. BMC Infect Dis. 2020. PMID: 32660552 Free PMC article. - Tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) in central nervous system inflammation.
Hoffmann O, Zipp F, Weber JR. Hoffmann O, et al. J Mol Med (Berl). 2009 Aug;87(8):753-63. doi: 10.1007/s00109-009-0484-x. Epub 2009 May 17. J Mol Med (Berl). 2009. PMID: 19449143 Review. - TRAIL limits excessive host immune responses in bacterial meningitis.
Hoffmann O, Priller J, Prozorovski T, Schulze-Topphoff U, Baeva N, Lunemann JD, Aktas O, Mahrhofer C, Stricker S, Zipp F, Weber JR. Hoffmann O, et al. J Clin Invest. 2007 Jul;117(7):2004-13. doi: 10.1172/JCI30356. J Clin Invest. 2007. PMID: 17571163 Free PMC article. - Activation of brain endothelium by Escherichia coli K1 virulence factor cglD promotes polymorphonuclear leukocyte transendothelial migration.
Zhang K, Shi MJ, Niu Z, Chen X, Wei JY, Miao ZW, Zhao WD, Chen YH. Zhang K, et al. Med Microbiol Immunol. 2019 Feb;208(1):59-68. doi: 10.1007/s00430-018-0560-3. Epub 2018 Aug 31. Med Microbiol Immunol. 2019. PMID: 30171337
References
- Abe, K. 2000. Therapeutic potential of neurotrophic factors and neural stem cells against ischemic brain injury. J. Cereb. Blood Flow Metab. 20:1393-1408. - PubMed
- Angstwurm, K., S. Reuss, D. Freyer, G. Arnold, U. Dirnagl, R. R. Schumann, and J. R. Weber. 2000. Induced hypothermia in experimental pneumococcal meningitis. J. Cereb. Blood Flow Metab. 20:834-838. - PubMed
- Angstwurm, K., J. R. Weber, A. Segert, W. Burger, M. Weih, D. Freyer, K. M. Einhaupl, and U. Dirnagl. 1995. Fucoidin, a polysaccharide inhibiting leukocyte rolling, attenuates inflammatory responses in experimental pneumococcal meningitis in rats. Neurosci. Lett. 191:1-4. - PubMed
- Anonymous. 1999. Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke 30:2752-2758. - PubMed
- Arditi, M., E. O. Mason, J. S. Bradley, T. Q. Tan, W. J. Barson, G. E. Schutze, E. R. Wald, L. B. Givner, K. S. Kim, R. Yogev, and S. L. Kaplan. 1998. Three-year multicenter surveillance of pneumococcal meningitis in children: clinical characteristics, and outcome related to penicillin susceptibility and dexamethasone use. Pediatrics 102:1087-1097. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources