In vivo dynamics of antigen-specific regulatory T cells not predicted from behavior in vitro - PubMed (original) (raw)
In vivo dynamics of antigen-specific regulatory T cells not predicted from behavior in vitro
Ludger Klein et al. Proc Natl Acad Sci U S A. 2003.
Abstract
Adoptive transfer of antigen-specific CD25+CD4+ regulatory T cells was used to analyze the stability of their phenotype, their behavior after immunization, and their mode of suppressing cotransferred naive T cells in vivo. We found that regulatory T cells maintained their phenotype in the absence of antigen, were not anergic in vivo, and proliferated as extensively as naive CD4+ T cells after immunization without losing their suppressive function in vivo and in vitro. In vivo, the expansion of cotransferred naive T cells was suppressed relatively late in the response such that regulatory T cells expressing mostly IL-10 but not IL-2 or IFN-gamma represented the dominant subset of cells. Our results reveal properties of regulatory T cells that were not predicted from in vitro studies.
Figures
Fig. 1.
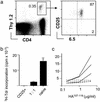
High frequency of HA-specific regulatory CD4+CD25+ T cells in _pgk_-HA × _TCR_-HA mice. (a) Expression of the transgenic TCR (mAb 6.5) versus CD25 on gated CD4 T cells from lymph nodes of _TCR_-HA single-transgenic versus _pgk_-HA × _TCR_-HA double-transgenic mice. Numbers in the dot plots indicate the percentage of gated cells within the respective quadrants. (b) CD25+ CD4 T cells from _pgk_-HA × _TCR_-HA mice were anergic and suppressed the proliferation of naive CD4 T cells from_TCR_-_HA rag_–/– mice in vitro. Sorted CD4+CD25+6.5+ cells from _pgk_-HA × _TCR_-HA mice and naive CD4+6.5+ T cells from _TCR_-_HA rag_–/– mice were incubated either alone or together (ratio 1:1) in the presence of BALB/c splenocytes and HA-peptide for 90 h. Proliferation was measured as incorporation of [3H]thymidine (3H-Tdr) added for the last 20 h.
Fig. 2.
Transferred CD4+CD25+6.5+ T cells retain their phenotypic and in vitro regulatory properties in the absence of antigen and suppress endogenous anti-HA-specific T cells after immunization. (a) CD4+CD25+6.5+ T cells (4 × 106) from Thy1.2+/+ _pgk_-HA × _TCR_-HA mice were transferred into BALB/c Thy1.1+/+ mice. Six days after transfer, the recipients were killed. Peripheral lymph nodes and spleen were pooled and stained for CD4, Thy1.2, CD25, and 6.5. The frequency of donor-derived cells among CD4 T cells is shown together with the sorting gate used for reisolation of cells. (Right) Purity of reisolated cells. (b) Reisolated Thy1.2+/+ cells 6 days after transfer were tested for their proliferative response after stimulation with HA-peptide and their suppressive potential when cocultured with naive cells as described for Fig. 1. (c) Recipients of 3 × 105 CD4+CD25+6.5+ T cells (dashed lines) or untreated BALB/c mice (solid lines) were immunized with HA-peptide (100 μg) in IFA. Eight days later, draining lymph node cells were harvested and stimulated in vitro with titrated amounts of HA-peptide for 90 h. Incorporation of [3H]thymidine (3H-Tdr) within the last 20 h was measured. The graph shows the data for three immunized mice of each group representative for three independent experiments.
Fig. 3.
Accumulation of CD4+CD25+6.5+ T cells or naive CD4+CD25–6.5+ T cells in the draining lymph nodes after immunization. (a) Thy1.1+/+ recipients of CD4+CD25+6.5+ T cells, CD4+CD25–6.5+ T cells, or both types of cells were immunized with HA-peptide as described for Fig. 2. Draining lymph node cells were harvested on day 8 after immunization and stained for CD4, Thy1.2, CD25, and 6.5. (Left) Numbers in the dot plots indicate the percentage of donor-derived cells among CD4+ T cells (mean of four animals). (Right) The dot plots show the expression of the transgenic TCR (6.5) versus CD25 on gated CD4+Thy1.2+ cells. Numbers within the dot plots indicate the percentage of gated cells in the respective quadrant (mean of four animals).
Fig. 4.
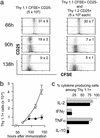
Proliferation of adoptively transferred CD4+CD25+6.5+ T cells or CD4+CD25–6.5+ T cells in the draining lymph nodes after immunization. CFSE-labeled CD4+CD25+6.5+ T cells from_pgk_-HA × _TCR_-HA mice (3 × 105) (Left) or CFSE-labeled naive CD4+CD25–6.5+ T cells from_TCR_-_HA rag_–/– mice (3 × 105) were transferred into BALB/c Thy1.1 mice. Two days later, recipients were immunized with 100 μg of HA-peptide in IFA. Controls were immunized with IFA without peptide. Mice were killed at the indicated time points after immunization, and draining lymph node cells were harvested and stained for CD4, Thy1.2, and CD25. The dot plots show the expression of CD25 versus CFSE fluorescence intensity on gated donor-derived cells (CD4+Thy1.2+). Numbered arrows within the dot plots (66 h) indicate the number of divisions of CFSE-labeled cells. Note that all dot plots (except controls, where ≈200 events are shown) show 800–1,000 CD4+Thy1.2+ events and thus do not represent the frequency of these cells among host CD4 T cells. (Right) Representative analysis of a cotransfer of 3 × 105 CFSE-labeled naive CD4+CD25–6.5+ T cells from_TCR_-_HA rag_–/– mice and 3 × 105 unlabeled CD4+CD25+6.5+ T cells from_pgk_-HA × _TCR_-HA mice into BALB/c Thy1.1 recipients.
Fig. 5.
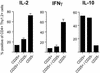
Cytokine production of transferred CD4+CD25+6.5+ T cells or CD4+CD25–6.5+ T cells. (a) Transferred into Thy1.2 recipients were 3 × 105 CD4+CD25+6.5+ T cells, CD4+CD25–6.5+ T cells, or both. Mice were immunized with HA-peptide in IFA, and draining lymph node cells were harvested 8 days after immunization. Cells were restimulated in vitro with phorbol 12-myristate 13-acetate/ionomycin for6hinthe presence of brefeldin A before surface staining for CD4 and Thy1.2, fixation, and intracellular staining for the indicated cytokine. The frequencies of cytokine-positive cells among gated CD4+Thy1.2+ draining lymph node cells of the indicated groups of animals are shown (for original data see Fig. 10).
Fig. 6.
Expansion, CD25 expression, and cytokine production after immunization of adoptively transferred naive CD4+CD25–6.5+ T cells in the presence or absence of CD4+CD25+6.5+ regulatory T cells. CFSE-labeled naive CD4+CD25–6.5+ T cells (5 × 105) sorted from Thy1.1+ _TCR_-HA mice (rag+/+) were adoptively transferred into BALB/c Thy1.2 recipients (Left) or BALB/c Thy1.2 recipients that had in addition received an equal number of CD4+CD25+6.5+ T cells from Thy1.2+ _pgk_-HA × _TCR_-HA mice (Right). Mice were immunized as described before, and draining lymph node cells were harvested at the indicated time points after immunization. (a) Expression of CD25 versus CFSE fluorescence intensity on gated (CD4+Thy1.1+) progeny of naive CD4+CD25–6.5+ T cells. Numbers within the upper quadrants indicate the frequency of CD25+ cells among CD4+Thy1.1+ cells (mean of four per group). Note the “smearing” into lower-division numbers in the presence of regulatory T cells (Right). (b) Absolute number in the draining lymph nodes of the progeny of naive CD4+CD25–6.5+ T cells after immunization in the presence (filled circles) or absence (open circles) of CD4+CD25+6.5+ regulatory T cells (mean of four per group). The number at 0 h (i.e., without immunization) was <0.1 × 105. (c) Draining lymph node cells were harvested on day 8 after immunization, stimulated in vitro with phorbol 12-myristate 13-acetate/ionomycin for 6 h in the presence of brefeldin A, and stained for CD4, Thy1.1, and the respective cytokine as indicated. The frequency of cytokine-producing cells among gated Thy1.1+CD4+ T cells is shown. Gray bars, recipients of naive CD4+CD25–6.5+ T cells alone; black bars, recipients of naive CD4+CD25–6.5+ T cells plus CD4+CD25+6.5+ regulatory T cells (mean of four per group). TNFα, tumor necrosis factor α.
Similar articles
- Induction of antigen-specific immunologic tolerance by in vivo and in vitro antigen-specific expansion of naturally arising Foxp3+CD25+CD4+ regulatory T cells.
Nishimura E, Sakihama T, Setoguchi R, Tanaka K, Sakaguchi S. Nishimura E, et al. Int Immunol. 2004 Aug;16(8):1189-201. doi: 10.1093/intimm/dxh122. Epub 2004 Jul 5. Int Immunol. 2004. PMID: 15237110 - Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance.
Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. Itoh M, et al. J Immunol. 1999 May 1;162(9):5317-26. J Immunol. 1999. PMID: 10228007 - A model of suppression of the antigen-specific CD4 T cell response by regulatory CD25+CD4 T cells in vivo.
Thorstenson KM, Herzovi L, Khoruts A. Thorstenson KM, et al. Int Immunol. 2005 Apr;17(4):335-42. doi: 10.1093/intimm/dxh213. Epub 2005 Feb 14. Int Immunol. 2005. PMID: 15710910 - Induction of antigen-specific regulatory T cells in the liver-draining celiac lymph node following oral antigen administration.
Hultkrantz S, Ostman S, Telemo E. Hultkrantz S, et al. Immunology. 2005 Nov;116(3):362-72. doi: 10.1111/j.1365-2567.2005.02236.x. Immunology. 2005. PMID: 16236126 Free PMC article. - CD4+ CD25+ [corrected] regulatory T cells render naive CD4+ CD25- T cells anergic and suppressive.
Qiao M, Thornton AM, Shevach EM. Qiao M, et al. Immunology. 2007 Apr;120(4):447-55. doi: 10.1111/j.1365-2567.2007.02544.x. Epub 2007 Jan 17. Immunology. 2007. PMID: 17244157 Free PMC article.
Cited by
- Origin and functions of pro-inflammatory cytokine producing Foxp3+ regulatory T cells.
Pandiyan P, Zhu J. Pandiyan P, et al. Cytokine. 2015 Nov;76(1):13-24. doi: 10.1016/j.cyto.2015.07.005. Epub 2015 Jul 10. Cytokine. 2015. PMID: 26165923 Free PMC article. Review. - CD4+CD25+ T regulatory cells, immunotherapy of cancer, and interleukin-2.
Antony PA, Restifo NP. Antony PA, et al. J Immunother. 2005 Mar-Apr;28(2):120-8. doi: 10.1097/01.cji.0000155049.26787.45. J Immunother. 2005. PMID: 15725955 Free PMC article. Review. - Incomplete depletion and rapid regeneration of Foxp3+ regulatory T cells following anti-CD25 treatment in malaria-infected mice.
Couper KN, Blount DG, de Souza JB, Suffia I, Belkaid Y, Riley EM. Couper KN, et al. J Immunol. 2007 Apr 1;178(7):4136-46. doi: 10.4049/jimmunol.178.7.4136. J Immunol. 2007. PMID: 17371969 Free PMC article. - Genomic definition of multiple ex vivo regulatory T cell subphenotypes.
Feuerer M, Hill JA, Kretschmer K, von Boehmer H, Mathis D, Benoist C. Feuerer M, et al. Proc Natl Acad Sci U S A. 2010 Mar 30;107(13):5919-24. doi: 10.1073/pnas.1002006107. Epub 2010 Mar 15. Proc Natl Acad Sci U S A. 2010. PMID: 20231436 Free PMC article. - Pathogenic T cells have a paradoxical protective effect in murine autoimmune diabetes by boosting Tregs.
Grinberg-Bleyer Y, Saadoun D, Baeyens A, Billiard F, Goldstein JD, Grégoire S, Martin GH, Elhage R, Derian N, Carpentier W, Marodon G, Klatzmann D, Piaggio E, Salomon BL. Grinberg-Bleyer Y, et al. J Clin Invest. 2010 Dec;120(12):4558-68. doi: 10.1172/JCI42945. Epub 2010 Nov 22. J Clin Invest. 2010. PMID: 21099113 Free PMC article.
References
- Modigliani, Y., Bandeira, A. & Coutinho, A. (1996) Immunol. Rev. 149, 155–120. - PubMed
- Le Douarin, N., Corbel, C., Bandeira, A., Thomas-Vaslin, V., Modigliani, Y., Coutinho, A. & Salaun, J. (1996) Immunol. Rev. 149, 35–53. - PubMed
- Sakaguchi, S., Sakaguchi, N., Shimizu, J., Yamazaki, S., Sakihama, T., Itoh, M., Kuniyasu, Y., Nomura, T., Toda, M. & Takahashi, T. (2001) Immunol. Rev. 182, 18–32. - PubMed
- Jordan, M. S., Riley, M. P., von Boehmer, H. & Caton, A. J. (2000) Eur. J. Immunol. 30, 136–144. - PubMed
- Jordan, M. S., Boesteanu, A., Reed, A. J., Petrone, A. L., Holenbeck, A. E., Lerman, M. A., Naji, A. & Caton, A. J. (2001) Nat. Immunol. 2, 301–306. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials