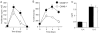OX40 (CD134) controls memory T helper 2 cells that drive lung inflammation - PubMed (original) (raw)
OX40 (CD134) controls memory T helper 2 cells that drive lung inflammation
Shahram Salek-Ardakani et al. J Exp Med. 2003.
Abstract
Asthma is caused by memory Th2 cells that often arise early in life and persist after repeated encounters with allergen. Although much is known regarding how Th2 cells develop, there is little information about the molecules that regulate memory Th2 cells after they have formed. Here we show that the costimulatory molecule OX40 is expressed on memory CD4 cells. In already sensitized animals, blocking OX40-OX40L interactions at the time of inhalation of aerosolized antigen suppressed memory effector accumulation in lung draining lymph nodes and lung, and prevented eosinophilia, airway hyperreactivity, mucus secretion, and Th2 cyto-kine production. Demonstrating that OX40 signals directly regulate memory T cells, antigen-experienced OX40-deficient T cells were found to divide initially but could not survive and accumulate in large numbers after antigen rechallenge. Thus, OX40-OX40L interactions are pivotal to the efficiency of recall responses regulated by memory Th2 cells.
Figures
Figure 1.
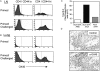
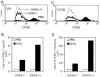
OX40 is expressed on memory and memory effector T cells. Groups of four C57BL/6 mice were immunized i.p. with OVA adsorbed to alum (primed). 25 d later mice were challenged by inhalation of nebulized OVA on 4 consecutive days (primed/challenged). (A) Peribronchial lymph nodes or (B) lungs were examined for expression of OX40 on CD4/CD44lo or CD4/CD44hi T cells. (C) Total numbers of OX40 positive CD4/CD44hi T cells (mean of 4 mice) were quantitated in lymph nodes from primed mice challenged with PBS (Alum-OVA/PBS), primed mice challenged with OVA (Alum-OVA/OVA), or unprimed mice challenged with OVA (Alum/OVA). (D) Lung sections were prepared 24 h after the last aerosol challenge and were stained with isotype matched control Ig (top) or anti-OX40 (bottom). A brown reaction product indicates OX40 staining. Similar results were seen in three experiments.
Figure 2.
Anti-OX40L suppresses memory T cell induced AHR and airway inflammation. (A) Experimental protocol for anti-OX40L administration. Unprimed control mice were injected i.p. with alum alone (Alum), while primed mice were sensitized with OVA adsorbed to alum (Alum-OVA). 25 d later, all mice were challenged with aerosolized OVA on 4 consecutive days (days 25–28; indicated by arrows). Either PBS (Group A: Alum/OVA), or control IgG (Group B: Alum-OVA/IgG-OVA) or anti-OX40L (Group C: Alum-OVA/RM134L-OVA) were administered i.p. on each challenge day. (B) 1–3 h after the last aerosol challenge, individual mice were assessed for AHR. Results are the mean percent change in Penh levels above baseline (saline-induced AHR), after exposure to increasing concentrations of inhaled methacholine. Values are calculated from four mice in each group per experiment. Similar results were seen in three experiments. (C) Total leukocyte numbers were enumerated in BAL at different times (0, 1, 2, 3, and 4 d) after exposure to aerosolized OVA (indicated by arrows). (D) Total numbers of eosinophils were calculated from differential stained BAL cytospins. Results are the mean number of cells ± SEM from two separate experiments with four mice per group in each experiment.
Figure 3.
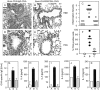
Anti-OX40L inhibits lung infiltration, goblet cell hyperplasia, mucus, serum IgE, and BAL Th2 cytokine production. Groups of mice were immunized and challenged as described in Fig. 2. 24 h after the final OVA aerosol challenge, lung tissue was stained with H&E (×100) for quantitation of inflammatory infiltrates (A and B) and periodic acid-Schiff (PAS, ×200; purple-red staining) to highlight the mucus-secreting cells (D and E), in sensitized and challenged animals receiving control Ig (A and D) or anti-OX40L (B and E). Sections were graded for inflammation severity (C) and mucus production (F) with groups A (white bars), B (black bars), and C (gray bars) corresponding to those described in Fig. 2. Results are the mean score ± SEM from four separate experiments with four mice per group in each experiment. 24 h after the final OVA aerosol challenge, sera were analyzed for OVA-specific IgE (G), and BAL were assessed for IL-4 (H), IL-5 (I), IL-9 (J), and IL-13 (K). Results are the mean values ± SEM from two separate experiments with four mice per group in each experiment.
Figure 4.
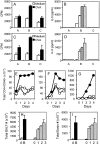
OX40 signals control memory T cell responses in secondary lymphoid organs. Mice were immunized and challenged as in Fig. 2. (A–D) 1 d after the last OVA challenge, lung (A and B), and lymph node cells (C and D) were cultured in medium alone or in the presence of increasing doses of OVA (10, 50, 100 μg/ml). (A and C) Proliferation at 72 h with 10 μg/ml OVA. (B and D) IL-5 production (Group A: Alum/OVA; Group B: Alum-OVA/IgG-OVA; Group C: Alum-OVA/RM134L-OVA). Results are the mean ± SEM from quadruplicate cultures and are representative of three experiments. Similar results were obtained for IL-13. (E–G) Peribronchial lymph node (E), lung (F), and BAL (G) cells from unimmunized and challenged mice (⋄), or OVA-immunized and challenged mice treated with control Ab (•) vs. anti-OX40L Ab (O), were harvested before (day 0) or on the indicated days after the first OVA challenge. T cells were stained for CD4 and OX40. The total numbers of OX40+ CD4 T cells were calculated from four mice in each group after gating on viable CD4+ T cells. Similar results were seen in three experiments. (H and I) Total leukocyte (H) and eosinophil (I) numbers in BAL recovered from mice given anti-OX40L throughout the aerosol challenge (day 0), or 1, 2, or 3 d after the initial aerosol. Results are the mean ± SEM from one experiment with four mice per group.
Figure 5.
Primed OX40−/− T cells do not survive efficiently in recall responses in vitro. OVA-specific Th2 memory cells were generated in vitro from wild-type (OX40+/+, closed symbols) or OX40-deficient (OX40−/−, open symbols) OT-II TCR transgenic mice as described in Materials and Methods. Primed T cells were restimulated with OVA peptide and APCs and proliferation (A) and survival (B) measured over 6 d. Cytokine production (C) was measured at 40 h. Data are means ± SEM from triplicate cultures, and representative of two separate experiments. Similar results were obtained if naive T cells were stimulated with antigen or anti-CD3 in primary cultures.
Figure 6.
Primed OX40−/− T cells do not accumulate efficiently in recall responses in vivo. OVA-specific Th2 memory cells were generated in vitro as in Fig. 5, from wild-type (OX40+/+) or OX40-deficient (OX40−/−) OT-II TCR transgenic mice. Primed T cells were labeled with CFSE and injected i.v. into naive C57BL/6 mice. Recipient mice were subsequently exposed to inhaled OVA or PBS on two consecutive days. 1 d after the last OVA challenge, peribronchial lymph node (A and B) and lung (C and D) were analyzed by flow cytometry for division (A and C) and accumulation (B and D) of transferred CFSE/Vα2 positive CD4 T cells. Data are representative of two separate experiments.
Figure 7.
Primed OX40-deficient T cells cannot induce pronounced airway inflammation. OVA-specific Th2 memory cells were generated in vitro as in Fig. 5, from wild-type (OX40+/+) or OX40-deficient (OX40−/−) OT-II TCR transgenic mice. Primed T cells were injected i.v. into naive C57BL/6 mice. Recipient mice were subsequently exposed to aerosolized OVA or PBS on two consecutive days. (A) Eosinophil numbers in BAL 24 h after the last OVA challenge. Results are mean ± SEM from four mice per group. (B–E) H&E stained lungs from mice receiving wild-type (B and C) or OX40-deficient T cells (D and E) after challenge with PBS (B and D) or OVA (C and E). (F) IL-4, IL-5, and IL-13 levels in BAL from mice with wt T cells challenged with PBS (Grp A), wt T cells challenged with OVA (Grp B), OX40−/− T cells challenged with OVA (Grp C). Similar results were seen in three separate experiments.
Figure 8.
OX40/OX40L interactions control both late secondary and tertiary recall responses to inhaled antigen. (A) Immunization protocol for secondary response of late memory T cells. Unprimed control mice were injected i.p. with alum alone (Alum), while primed mice were sensitized with OVA adsorbed to alum (Alum-OVA). 60 d later, all mice were challenged with aerosolized OVA on 4 consecutive days (days 60–64; indicated by arrows). Either PBS (Group A: Alum/OVA), or control IgG (Group B: Alum-OVA/IgG-OVA) or anti-OX40L (Group C: Alum-OVA/RM134L-OVA) were administered i.p. on each challenge day (indicated by filled symbols). (D) Immunization protocol for tertiary response of late memory T cells. Unprimed control mice were injected i.p. with alum alone (Alum), while primed mice were sensitized with OVA adsorbed to alum (Alum-OVA). On days 25–29 all mice were challenged in a secondary response with aerosolized OVA. 91 d later (day 120), all mice were challenged in a tertiary response with aerosolized OVA on 4 consecutive days (days 120–124; indicated by arrows). Either PBS (Group A: Alum/OVA), or control IgG (Group B: Alum-OVA/IgG-OVA) or anti-OX40L (Group C: Alum-OVA/RM134L-OVA) were administered i.p. on each challenge day (indicated by filled symbols). 1 d after the last challenge mice were killed and airway eosinophilia and Th2 cytokine production determined. Total leukocyte (B and E) and eosinophil (C and F) numbers were enumerated in BAL from mice in protocol A (B and C) and protocol D (E and F) respectively. Analysis of Th2 cytokines showed the same profile as cell infiltration (unpublished data). Individual responses of four mice in each group are shown.
Similar articles
- Development of allergic inflammation in a murine model of asthma is dependent on the costimulatory receptor OX40.
Jember AG, Zuberi R, Liu FT, Croft M. Jember AG, et al. J Exp Med. 2001 Feb 5;193(3):387-92. doi: 10.1084/jem.193.3.387. J Exp Med. 2001. PMID: 11157058 Free PMC article. - OX40 blockade inhibits house dust mite driven allergic lung inflammation in mice and in vitro allergic responses in humans.
Burrows KE, Dumont C, Thompson CL, Catley MC, Dixon KL, Marshall D. Burrows KE, et al. Eur J Immunol. 2015 Apr;45(4):1116-28. doi: 10.1002/eji.201445163. Epub 2015 Jan 21. Eur J Immunol. 2015. PMID: 25545270 - Constitutive OX40/OX40 ligand interaction induces autoimmune-like diseases.
Murata K, Nose M, Ndhlovu LC, Sato T, Sugamura K, Ishii N. Murata K, et al. J Immunol. 2002 Oct 15;169(8):4628-36. doi: 10.4049/jimmunol.169.8.4628. J Immunol. 2002. PMID: 12370402 - OX40-OX40 ligand interaction in T-cell-mediated immunity and immunopathology.
Ishii N, Takahashi T, Soroosh P, Sugamura K. Ishii N, et al. Adv Immunol. 2010;105:63-98. doi: 10.1016/S0065-2776(10)05003-0. Adv Immunol. 2010. PMID: 20510730 Review. - The significance of OX40 and OX40L to T-cell biology and immune disease.
Croft M, So T, Duan W, Soroosh P. Croft M, et al. Immunol Rev. 2009 May;229(1):173-91. doi: 10.1111/j.1600-065X.2009.00766.x. Immunol Rev. 2009. PMID: 19426222 Free PMC article. Review.
Cited by
- Triclosan Induces Thymic Stromal Lymphopoietin in Skin Promoting Th2 Allergic Responses.
Marshall NB, Lukomska E, Long CM, Kashon ML, Sharpnack DD, Nayak AP, Anderson KL, Jean Meade B, Anderson SE. Marshall NB, et al. Toxicol Sci. 2015 Sep;147(1):127-39. doi: 10.1093/toxsci/kfv113. Epub 2015 Jun 5. Toxicol Sci. 2015. PMID: 26048654 Free PMC article. - Nitric oxide induces CD4+CD25+ Foxp3 regulatory T cells from CD4+CD25 T cells via p53, IL-2, and OX40.
Niedbala W, Cai B, Liu H, Pitman N, Chang L, Liew FY. Niedbala W, et al. Proc Natl Acad Sci U S A. 2007 Sep 25;104(39):15478-83. doi: 10.1073/pnas.0703725104. Epub 2007 Sep 17. Proc Natl Acad Sci U S A. 2007. PMID: 17875988 Free PMC article. - A therapeutic OX40 agonist dynamically alters dendritic, endothelial, and T cell subsets within the established tumor microenvironment.
Pardee AD, McCurry D, Alber S, Hu P, Epstein AL, Storkus WJ. Pardee AD, et al. Cancer Res. 2010 Nov 15;70(22):9041-52. doi: 10.1158/0008-5472.CAN-10-1369. Epub 2010 Nov 2. Cancer Res. 2010. PMID: 21045144 Free PMC article. - Type 2 cytokine responses: regulating immunity to helminth parasites and allergic inflammation.
Henry EK, Inclan-Rico JM, Siracusa MC. Henry EK, et al. Curr Pharmacol Rep. 2017 Dec;3(6):346-359. doi: 10.1007/s40495-017-0114-1. Epub 2017 Oct 19. Curr Pharmacol Rep. 2017. PMID: 29399438 Free PMC article. - Impaired induction of allergic lung inflammation by Alternaria alternata mutant MAPK homologue Fus3.
Kim HK, Baum R, Lund S, Khorram N, Yang SL, Chung KR, Doherty TA. Kim HK, et al. Exp Lung Res. 2013 Nov;39(9):399-409. doi: 10.3109/01902148.2013.835009. Epub 2013 Oct 8. Exp Lung Res. 2013. PMID: 24102366 Free PMC article.
References
- Wills-Karp, M. 1999. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu. Rev. Immunol. 17:255–281. - PubMed
- Umetsu, D.T., J.J. McIntire, O. Akbari, C. Macaubas, and R.H. DeKruyff. 2002. Asthma: an apidemic of dysregulated immunity. Nat. Immunol. 3:715–720. - PubMed
- Croft, M., L.M. Bradley, and S.L. Swain. 1994. Naive versus memory CD4 T cell response to antigen. Memory cells are less dependent on accessory cell costimulation and can respond to many antigen-presenting cell types including resting B cells. J. Immunol. 152:2675–2685. - PubMed
- Byrne, J.A., J.L. Butler, and M.D. Cooper. 1988. Differential activation requirements for virgin and memory T cells. J. Immunol. 141:3249–3257. - PubMed
- Luqman, M., and K. Bottomly. Activation requirements for CD4+ T cells differing in CD45R expression. J. Immunol. 149:2300–2306. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials