Canonical Wnt signals are essential for homeostasis of the intestinal epithelium - PubMed (original) (raw)
Canonical Wnt signals are essential for homeostasis of the intestinal epithelium
Daniel Pinto et al. Genes Dev. 2003.
Abstract
To assess the critical role of Wnt signals in intestinal crypts, we generated transgenic mice ectopically expressing Dickkopf1 (Dkk1), a secreted Wnt inhibitor. We find that epithelial proliferation is greatly reduced coincidentally with the loss of crypts. Although enterocyte differentiation appears unaffected, secretory cell lineages are largely absent. Disrupted intestinal homeostasis is reflected by an absence of nuclear beta-catenin, inhibition of c-myc expression, and subsequent up-regulation of p21CIP1/WAF1. Thus, our data are the first to establish a direct requirement for Wnt ligands in driving proliferation in the intestinal epithelium, and also define an unexpected role for Wnts in controlling secretory cell differentiation.
Figures
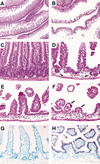
Figure 1.
Dkk1 ectopic expression deleteriously affects crypt–villus organization. Representative sections of the small intestine from wild-type (A,C) and transgenic (B,D_–_F) adult animals stained with hematoxylin/eosin. The number and the size of crypts and villi are drastically reduced in the transgenic mucosa to rare residual crypt-like structures (arrowheads in D) or few residual villi (arrows in_F_). In situ hybridization analysis of small intestine sections from wild-type (G) and transgenic (H) mice hybridized with a Dkk1 antisense RNA probe. Note that Dkk1 is only expressed in the transgenic epithelium. Original magnification: A,B, 2×;C_–_H, 20×.
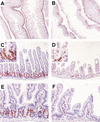
Figure 2.
Intestinal cell proliferation is inhibited in Dkk1 transgenic mice. Immunohistochemical analysis of small intestine sections from wild-type (A,C) or transgenic (B,D) adult mice stained with an antibody against proliferating cell antigen Ki-67, and wild-type (E) or transgenic (F) adult mice labeled with BrdU and stained with an anti-BrdU antibody. Note that proliferative cells are abundantly present in regularly distributed crypts flanking the villi from the wild-type intestine (C, inset), but are absent from patchy cryptless areas in the transgenic intestine, persisting only in scarce residual crypts (D, inset). Original magnification: A,B, 2×; C,D, 10×; insets, 60×; E,F, 20×.
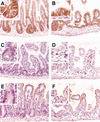
Figure 3.
Nuclear β-catenin and related target genes expression in transgenic intestinal mucosa. Immunohistochemical analysis of small intestine sections from wild-type (A,C,E) and transgenic (B,D,F) adult mice stained with antibodies against β-catenin (A,B), Enc-1 (C,D), and AP (E,F). Staining of β-catenin shows accumulation in the nucleus of cells at the crypt base (A and_inset_), but is absent from the nucleus of cells of the lining epithelium in the cryptless regions (B and inset). Staining indicates that Enc-1 cytoplasmic expression restricted to the crypt cells (C and inset) is completely absent from the intervillus lining epithelium (D and inset). Staining reveals that AP expression at the apical domain of the villus-associated cells (E and_inset_) is found in the cells of the lining epithelium in cryptless areas (F and inset). Original magnification:A_–_F, 20×; insets, 60×.
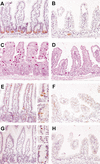
Figure 4.
Secretory cell lineage is depleted in Dkk1 transgenic intestinal epithelium. Representative sections of the small intestine from wild-type (A,C,E,G) and transgenic (B,D,F,H) adult animals stained for representation of secretory cell types: anti-lysozyme antibody (Paneth cells;A,B), periodic acid/Schiff (goblet cells; C,D), anti-synaptophysin (enteroendocrine cells and nerve fibers; E,F), and anti-Math1 antibody (all three types and common progenitors; G,H). Note that all three secretory cell types and their Math1-positive common progenitors (G, bottom inset) are absent from cryptless areas of the transgenic epithelium (except in residual crypts). Original magnification:A_–_H, 20×; insets, 40×.
Figure 5.
Complementary pattern of expression for c-Myc and p21CIP1/WAF1 in wild-type and transgenic intestinal epithelium. Representative sections of the small intestine from wild-type (A,B,E,F) and transgenic (C,D,G,H) adult animals stained with an anti-c-myc antibody (A_–_D) or an anti-p21CIP1/WAF1 antibody (E_–_H). The arrows in B and F indicate Paneth cells displaying a reverse nuclear staining for c-Myc and p21CIP1/WAF1. The arrowheads in D and H show that p21CIP1/WAF1 is expressed in the nucleus of the cells lining the intervillus epithelium contrary to c-Myc. Original magnification:A,C,E,G, 20×; B,D,F,H, 40×.
References
- Andl T., Reddy, S.T., Gaddapara, T., and Millar, S.E. 2002. WNT signals are required for the initiation of hair follicle development. Dev. Cell 2: 643–653. - PubMed
- Bafico A., Liu, G., Yaniv, A., Gazit, A., and Aaronson, S.A. 2001. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat. Cell Biol. 3: 683–686. - PubMed
- Batlle E., Henderson, J.T., Beghtel, H., van den Born, M.M., Sancho, E., Huls, G., Meeldijk, J., Robertson, J., van de Wetering, M., Pawson, T., et al. 2002. β-Catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell 111: 251–263. - PubMed
- Bienz M. and Clevers, H. 2000. Linking colorectal cancer to Wnt signaling. Cell 103: 311–320. - PubMed
- Booth C., Brady, G., and Potten, C.S. 2002. Crowd control in the crypt. Nat. Med. 8: 1360–1361. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases