Segregation of amphetamine reward and locomotor stimulation between nucleus accumbens medial shell and core - PubMed (original) (raw)
Segregation of amphetamine reward and locomotor stimulation between nucleus accumbens medial shell and core
Laurie H L Sellings et al. J Neurosci. 2003.
Abstract
Convergent evidence suggests that amphetamine (AMPH) exerts its rewarding and locomotor stimulating effects via release of dopamine in the nucleus accumbens. However, there is no consensus as to the relative contributions of core and medial shell subregions to these effects. Moreover, the literature is based primarily on intracranial administration, which cannot fully mimic the drug distribution achieved by systemic administration. In the present study, the effects of bilateral 6-hydroxydopamine lesions of the accumbens core or medial shell on rewarding and locomotor stimulating effects of systemically administered amphetamine (0.75 mg/kg, i.p.) were examined in a conditioned place preference (CPP) procedure relying solely on tactile cues (floor texture). Residual dopamine innervation was quantified by [125I]-RTI-55 binding to the dopamine transporter. When lesions were performed before the conditioning phase, AMPH-induced locomotor stimulation and CPP magnitude were positively correlated with residual dopamine transporter binding in core and medial shell, respectively. Medial shell lesions did not affect morphine CPP, arguing that a sensory or mnemonic deficit was not responsible for the lesion-induced reduction in AMPH CPP. Medial shell lesions performed between the conditioning phase and the test day reduced the expression of amphetamine CPP. These results suggest that after systemic amphetamine administration, rewarding and locomotor stimulating effects of the drug are anatomically dissociated within the nucleus accumbens: the medial shell contributes to rewarding effects, whereas the core contributes to behavioral activation.
Figures
Figure 1.
Experimental design of experiments 1, 2 and 3. Vehicle or 6-OHDA infusions were given at the time indicated by the arrows. In experiments 1 and 3, rats received infusions into either core or medial shell, depending on group (filled arrows). In experiment 2, only medial shell was targeted (white arrow). During the conditioning phase, each rat received saline and a drug (AMPH or morphine, dose as indicated) on alternating days (see Materials and Methods). IP, Intraperitoneal.
Figure 2.
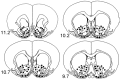
Histological changes associated with infusion of vehicle (A) or 6-OHDA (B) into the medial shell region of the NAcc. Representative 20 μm Nissl-stained sections are shown ∼0.1 mm caudal to the site of injection (10.2 mm anterior to interaural zero). 6-OHDA infusion resulted in disruption of normal tissue morphology local to the infusion site (B, black arrow). Much less disruption of normal tissue morphology occurred in rats infused with vehicle. Scale bars, 50 μm. Anterior commissure is indicated by white arrows.
Figure 3.
Autoradiographic images of [125I]RTI-55 binding to DAT in animals from core-lesioned, medial shell-lesioned, and sham-operated groups (experiment 3). Because binding was similar between groups that received vehicle in core and medial shell, the latter group has been omitted. Numbers designate distance anterior to interaural zero (in millimeters). Radioligand binding was obtained at a nonsaturating concentration of radioligand and is expressed as attomol per milligram of tissue. Arrows refer to the core subregion. Arrowheads (pointing upward) refer to the medial shell subregion. In most rats, core 6-OHDA lesions were less anatomically selective than shown here (see Fig. 5).
Figure 4.
Locations of sampled [125I]RTI-55 binding in nucleus accumbens core, medial shell, ventral shell, ventral caudate-putamen, and olfactory tubercle. Each rat was sampled at four anterior-posterior levels. Numbers are distances (in millimeters) anterior to interaural zero. Sampling areas were circles of 0.3 mm diameter. Three samples per side per structure were taken at each level, except for ventral shell, where one sample per side was taken at level 11.2 and two per side at all other levels. Adapted from Paxinos and Watson (1997).
Figure 5.
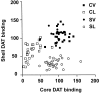
Relationship of DAT labeling in nucleus accumbens core versus medial shell. Data are pooled from experiments 1 and 3 (n = 98 rats). DAT labeling was performed by [125I]RTI-55 autoradiography and expressed as a percentage of the mean value of the core-vehicle group for core 6-OHDA animals, or the shell-vehicle group for the shell 6-OHDA group. Correlational analysis revealed a weak but significant relationship between core and medial shell binding (r = 0.30; p < 0.005). CV, Core vehicle; CL, core lesioned; SV, medial shell vehicle; SL, medial shell lesion.
Figure 6.
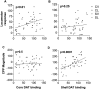
Effect of bilateral 6-OHDA infusion into either NAcc core or medial shell on AMPH-induced locomotor response and CPP (experiment 1). Rats (n = 10–14 per group) were allowed 7–11 d recovery after stereotaxic surgery before conditioning with AMPH (0.75 mg/kg, i.p.). Locomotor responses are expressed for each rat as the difference between the mean distance moved (in meters) during conditioning sessions with AMPH versus with saline. CPP magnitude is the difference between time spent on the drug-paired and saline-paired textures during the 600 sec test. DAT labeling in core or medial shell is expressed as percentage DAT binding of sham-lesioned groups. Saline locomotor scores, in meters, were 134 ± 7 in the core vehicle group, 152 ± 11 in the core 6-OHDA group, 154 ± 10 in the shell vehicle group, and 153 ± 11 in the shell 6-OHDA group. Locomotor responses (AMPH-saline) correlated significantly with DAT binding in NAcc core but not in NAcc medial shell. Conversely, CPP magnitude correlated significantly with DAT binding in medial shell but not core. To visualize the association of each drug response to core or medial shell [125I]RTI-55 labeling, the predicted contribution of the irrelevant brain structure was subtracted from the _y_-axis variables using the calculated multiple linear regression equation. Significant linear associations (shown by p values) are evident in A and D. CV, Core vehicle; CL, core lesioned; SV, medial shell vehicle; SL, medial shell lesion.
Figure 7.
Effect of 6-OHDA lesions of NAcc medial shell on morphine and AMPH CPP (experiment 2). Stereotaxic surgery was performed 7–11 d before the first conditioning day. CPP magnitudes (mean ± SEM) for morphine (10 mg/kg, i.p.) or AMPH (0.75 mg/kg, i.p.) were calculated as the difference between the time spent on the drug-paired and saline-paired sides (n = 10–12 rats per group). Because the data were not normally distributed, Mann–Whitney U tests were applied to predetermined comparisons. NS, Nonsignificant; *p < 0.02 versus corresponding sham-lesioned group (unprotected tests).
Figure 8.
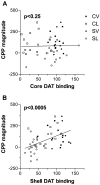
Effect of NAcc core and medial shell lesions on the expression of AMPH CPP (experiment3). Rats (n = 10–19 per group) received bilateral infusion of either 6-OHDA or vehicle into either NAcc core or medial shell after conditioning with AMPH and before CPP testing. Degree of DAT depletion in core or medial shell is expressed as percentage DAT binding of control. To visualize the association of each drug response to core or medial shell [125I]RTI-55 labeling, the predicted contribution of the irrelevant brain structure was subtracted from the _y_-axis variables using the calculated multiple linear regression equation. CPP magnitude correlated significantly with DAT binding in NAcc medial shell (B) but not with DAT binding in NAcc core (A). CV, Core vehicle; CL, core lesioned; SV, medial shell vehicle; SL, medial shell lesion.
Similar articles
- 6-Hydroxydopamine lesions of nucleus accumbens core abolish amphetamine-induced conditioned activity.
Sellings LH, Clarke PB. Sellings LH, et al. Synapse. 2006 May;59(6):374-7. doi: 10.1002/syn.20247. Synapse. 2006. PMID: 16463400 - Effect of ventral tegmental 6-hydroxydopamine lesions on the locomotor stimulant action of nicotine in rats.
Louis M, Clarke PB. Louis M, et al. Neuropharmacology. 1998 Dec;37(12):1503-13. doi: 10.1016/s0028-3908(98)00151-8. Neuropharmacology. 1998. PMID: 9886673 - Characterization of dopamine-dependent rewarding and locomotor stimulant effects of intravenously-administered methylphenidate in rats.
Sellings LH, McQuade LE, Clarke PB. Sellings LH, et al. Neuroscience. 2006 Sep 1;141(3):1457-68. doi: 10.1016/j.neuroscience.2006.04.040. Epub 2006 Jun 6. Neuroscience. 2006. PMID: 16753267 - Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies.
McBride WJ, Murphy JM, Ikemoto S. McBride WJ, et al. Behav Brain Res. 1999 Jun;101(2):129-52. doi: 10.1016/s0166-4328(99)00022-4. Behav Brain Res. 1999. PMID: 10372570 Review. - Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex.
Ikemoto S. Ikemoto S. Brain Res Rev. 2007 Nov;56(1):27-78. doi: 10.1016/j.brainresrev.2007.05.004. Epub 2007 May 17. Brain Res Rev. 2007. PMID: 17574681 Free PMC article. Review.
Cited by
- Using In Vitro Electrophysiology to Screen Medications: Accumbal Plasticity as an Engram of Alcohol Dependence.
Renteria R, Jeanes ZM, Mangieri RA, Maier EY, Kircher DM, Buske TR, Morrisett RA. Renteria R, et al. Int Rev Neurobiol. 2016;126:441-65. doi: 10.1016/bs.irn.2016.02.018. Epub 2016 Mar 24. Int Rev Neurobiol. 2016. PMID: 27055622 Free PMC article. - Gene expression differences in mice divergently selected for methamphetamine sensitivity.
Palmer AA, Verbitsky M, Suresh R, Kamens HM, Reed CL, Li N, Burkhart-Kasch S, McKinnon CS, Belknap JK, Gilliam TC, Phillips TJ. Palmer AA, et al. Mamm Genome. 2005 May;16(5):291-305. doi: 10.1007/s00335-004-2451-8. Mamm Genome. 2005. PMID: 16104378 - Neurochemical and behavioral consequences of widespread gene knockdown in the adult mouse brain by using nonviral RNA interference.
Thakker DR, Natt F, Hüsken D, Maier R, Müller M, van der Putten H, Hoyer D, Cryan JF. Thakker DR, et al. Proc Natl Acad Sci U S A. 2004 Dec 7;101(49):17270-5. doi: 10.1073/pnas.0406214101. Epub 2004 Nov 29. Proc Natl Acad Sci U S A. 2004. PMID: 15569935 Free PMC article. - Nicotine-conditioned single-trial place preference: selective role of nucleus accumbens shell dopamine D1 receptors in acquisition.
Spina L, Fenu S, Longoni R, Rivas E, Di Chiara G. Spina L, et al. Psychopharmacology (Berl). 2006 Mar;184(3-4):447-55. doi: 10.1007/s00213-005-0211-4. Epub 2005 Dec 10. Psychopharmacology (Berl). 2006. PMID: 16341849 - Impact of juvenile chronic stress on adult cortico-accumbal function: Implications for cognition and addiction.
Watt MJ, Weber MA, Davies SR, Forster GL. Watt MJ, et al. Prog Neuropsychopharmacol Biol Psychiatry. 2017 Oct 3;79(Pt B):136-154. doi: 10.1016/j.pnpbp.2017.06.015. Epub 2017 Jun 19. Prog Neuropsychopharmacol Biol Psychiatry. 2017. PMID: 28642080 Free PMC article. Review.
References
- Acquas E, Di Chiara G ( 1994) D1 receptor blockade stereospecifically impairs the acquisition of drug-conditioned place preference and place aversion. Behav Pharmacol 5: 555–569. - PubMed
- Archer T, Fredriksson A, Jonsson G, Lewander T, Mohammed AK, Ross SB, Soderberg U ( 1986) Central noradrenaline depletion antagonizes aspects of d-amphetamine-induced hyperactivity in the rat. Psychopharmacology 88: 141–146. - PubMed
- Bardo MT, Bevins RA ( 2000) Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology 153: 31–43. - PubMed
- Bardo MT, Valone JM, Bevins RA ( 1999) Locomotion and conditioned place preference produced by acute intravenous amphetamine: role of dopamine receptors and individual differences in amphetamine self-administration. Psychopharmacology 143: 39–46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources