The role(s) of lipophosphoglycan (LPG) in the establishment of Leishmania major infections in mammalian hosts - PubMed (original) (raw)
The role(s) of lipophosphoglycan (LPG) in the establishment of Leishmania major infections in mammalian hosts
Gerald F Späth et al. Proc Natl Acad Sci U S A. 2003.
Abstract
The abundant cell surface glycolipid lipophosphoglycan (LPG) was implicated in many steps of the Leishmania infectious cycle by biochemical tests. The presence of other abundant surface or secreted glycoconjugates sharing LPG domains, however, has led to uncertainty about the relative contribution of LPG in vivo. Here we used an Leishmania major lpg1- mutant, which lacks LPG alone and shows attenuated virulence, to dissect the role of LPG in the establishment of macrophage infections in vivo. lpg1- was highly susceptible to human complement, had lost the ability to inhibit phagolysosomal fusion transiently, and was oxidant sensitive. Studies of mouse mutants defective in relevant defense mechanisms confirmed the role of LPG in oxidant resistance but called into question the importance of transient inhibition of phagolysosomal fusion for Leishmania macrophage survival. Moreover, the limited lytic activity of mouse complement appears to be an ineffective pathogen defense mechanism in vitro and in vivo, unlike human hosts. In contrast, lpg1- parasites bound C3b and resisted low pH and proteases normally, entered macrophages efficiently and silently, and continued to inhibit host-signaling pathways. These studies illustrate the value of mechanistic approaches focusing on both parasite and host defense pathways in dissecting the specific biological roles of complex virulence factors such as LPG.
Figures
Fig. 5.
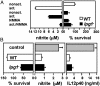
Intact LPG is not required for quiet entry (without macrophage activation) or for inhibition of macrophage activation. (A)_lpg1_- promastigotes do not activate PEMs. PEMs were infected with purified C3b-opsonized WT (open bars) or_lpg1_- (filled bars) metacyclics. As indicated, the PEMs were incubated 12 h before infection with 100 μM NMMA, 100 ng/ml LPS plus 100 units/ml IFN-γ, or NMMA plus LPS plus IFN-γ. NO production was measured after 24 h and parasite survival was monitored; only the 5-day survival data are shown. Two independent experiments were performed; bars show standard deviations of a representative triplicate experiment. (B)_lpg1_- parasites retain the ability to inhibit PEM activation. Purified serum-opsonized WT (open bars) or_lpg1_- (filled bars) metacyclics were allowed to infect PEMs for 6 h, and LPS plus IFN-γ were added for another 24 h. Controls (gray bars) were mock-infected PEMs. NO-derived nitrite in culture supernatants was determined by the Griess reaction (46). IL-12 p40 levels were determined in the PEM culture supernatants by an ELISA capture method (PharMingen). Two experiments were performed; the average and standard deviation for one with triplicate samples are shown.
Fig. 1.
_lpg1_- metacyclic promastigotes are sensitive to human but not mouse complement. (A) Flow cytometric analysis of lysis by human serum. Metacyclic WT, _lpg1_-, and_lpg1_-/+LPG1 lines were incubated in the presence (shaded) or absence (open) of 2% human serum for 30 min in the presence of propidium iodide and then subjected to flow cytometry. (B) Infections of normal and C5-deficient mice. WT (○) or _lpg1_- (▵) metacyclics (105) were inoculated into the footpad of isogenic WT (Upper) or C5-deficient (Lower) mice, and lesion formation was followed. The average and standard deviation are shown for groups of five mice; this experiment is representative of three independent tests.
Fig. 2.
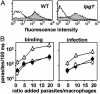
_lpg1_- metacyclic promastigote parasites are opsonized efficiently by C3b and can invade macrophages. (A) Opsonization by C3b. WT (Left)or _lpg1_- (Right) metacyclics were treated with normal (shaded) or heat-inactivated (open) mouse serum, and deposition of C3b was quantitated by flow cytometry. Controls in which anti-C3b was omitted gave results similar to that obtained with heat-inactivated serum (data not shown). (B) Attachment and entry of opsonized metacyclics into macrophages (mφ). Metacyclics were opsonized with C5-deficient mouse serum and incubated with cultured PEMs (3 × 105 per well; WT, ○; _lpg1_-, ▵; and_lpg1_-/+LPG1, •) at the indicated moi. For attachment (Left), parasites were incubated for 20 min at 4°C; for uptake (Right) parasites were incubated for 2 h more at 37°C. Free parasites were removed by washing with cold PBS, and cells were fixed with 3.5% paraformaldehyde, stained, and counted. The bars show the standard deviations of three replicates and are representative of at least three independent experiments.
Fig. 3.
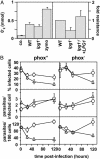
_lpg1_- promastigotes are susceptible to oxidative stress. (A) _lpg1_- and WT Leishmania induce comparable PEM oxidative bursts (Left). PEMs were treated with zymosan or incubated with 4-day stationary-phase WT or_lpg1_- promastigotes for 90 min at 37°C, and superoxide production was determined (45). The average and standard deviation of triplicate determinations are shown, representative of two independent experiments. _lpg1_- parasites are more susceptible to oxidative stress (Right). The relative resistance of WT, _lpg1_-, and _lpg1_-/+LPG1 promastigotes to oxidants generated by the xanthine–xanthine oxidase system is shown. An EC50 was calculated, data were normalized to WT, and the average and standard deviation are shown. (B)_lpg1_- survives normally in infections of_phox_- macrophages. Infection efficiency (% infected cells,Top), intracellular growth (parasites per infected cell,Middle), and parasite survival (parasites per 100 cells,Bottom) of C3b-opsonized WT and _lpg1_- metacyclics in PEMs derived from C57BL/6 control (phox+) or isogenic oxidant-deficient (_phox_-) mice are shown. Three independent experiments were performed; bars show the standard deviations of one representative triplicate experiment.
Fig. 4.
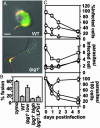
Release and effects of PGs in infected macrophages. (A) Release of PGs. PEMs were incubated (20 min at 4°C) with serum-opsonized metacyclic WT or lpg1_- promastigotes (moi of 10 or 3, respectively, to normalize for initial uptake; see Fig. 2_B) and then incubated for up to3hat37°C. Cells were fixed with 3.5% paraformaldehyde (5 min at RT), permeabilized with 100% ethanol (15 min at 4°C), incubated for 20 min at 37°C with anti-PG antibody (1:500 dilution of WIC79.3) and anti-tubulin antibody (1:2,000 dilution of anti-tubulin primary antibodies), washed with PBS, and incubated further with an FITC-conjugated goat anti-mouse IgG and Texas-red-conjugated donkey anti-rabbit IgG (each at 1:200 dilution; Jackson Immunochemicals). Exposures were 0.66 s for WT-infected macrophages and 0.25 s for_lpg1_--infected macrophages (because PG levels are much higher in WT Leishmania). (Scale bar = 5 μm.) (B and_C_) _lpg1_- metacyclic parasites are defective in inhibition of phagolysosomal fusion and survival. Phagolysosomal fusion was quantitated by labeling of parasitophorous vacuoles with FITC-dextran, loaded previously into the lysosomal compartment (see Materials and Methods). The ratio of infecting _lpg1_- to PEMs was varied, and the effect on phagolysosomal fusion (B, 3 h postinfection) or parasite survival (C) was determined in triplicate. Purified C3-opsonized metacyclic _lpg1_- promastigotes were infected at an moi of 3 (low) or 10 (high); at the 2-h time point, 1.4 or 7.4 intracellular parasites per infected macrophage were obtained, respectively. The bars indicate standard deviations; one experiment is shown, which is representative of three independent experiments.
Similar articles
- Lipophosphoglycan is a virulence factor distinct from related glycoconjugates in the protozoan parasite Leishmania major.
Späth GF, Epstein L, Leader B, Singer SM, Avila HA, Turco SJ, Beverley SM. Späth GF, et al. Proc Natl Acad Sci U S A. 2000 Aug 1;97(16):9258-63. doi: 10.1073/pnas.160257897. Proc Natl Acad Sci U S A. 2000. PMID: 10908670 Free PMC article. - Lipophosphoglycan is not required for infection of macrophages or mice by Leishmania mexicana.
Ilg T. Ilg T. EMBO J. 2000 May 2;19(9):1953-62. doi: 10.1093/emboj/19.9.1953. EMBO J. 2000. PMID: 10790362 Free PMC article. - Leishmania Lipophosphoglycan Triggers Caspase-11 and the Non-canonical Activation of the NLRP3 Inflammasome.
de Carvalho RVH, Andrade WA, Lima-Junior DS, Dilucca M, de Oliveira CV, Wang K, Nogueira PM, Rugani JN, Soares RP, Beverley SM, Shao F, Zamboni DS. de Carvalho RVH, et al. Cell Rep. 2019 Jan 8;26(2):429-437.e5. doi: 10.1016/j.celrep.2018.12.047. Cell Rep. 2019. PMID: 30625325 Free PMC article. - Leishmania lipophosphoglycan: how to establish structure-activity relationships for this highly complex and multifunctional glycoconjugate?
Forestier CL, Gao Q, Boons GJ. Forestier CL, et al. Front Cell Infect Microbiol. 2015 Jan 21;4:193. doi: 10.3389/fcimb.2014.00193. eCollection 2014. Front Cell Infect Microbiol. 2015. PMID: 25653924 Free PMC article. Review. - Adversarial relationship between the leishmania lipophosphoglycan and protein kinase C of host macrophages.
Turco SJ. Turco SJ. Parasite Immunol. 1999 Dec;21(12):597-600. doi: 10.1046/j.1365-3024.1999.00266.x. Parasite Immunol. 1999. PMID: 10583861 Review.
Cited by
- Proteinases as virulence factors in Leishmania spp. infection in mammals.
Silva-Almeida M, Pereira BA, Ribeiro-Guimarães ML, Alves CR. Silva-Almeida M, et al. Parasit Vectors. 2012 Aug 7;5:160. doi: 10.1186/1756-3305-5-160. Parasit Vectors. 2012. PMID: 22871236 Free PMC article. - Galectin-1 on cervical epithelial cells is a receptor for the sexually transmitted human parasite Trichomonas vaginalis.
Okumura CY, Baum LG, Johnson PJ. Okumura CY, et al. Cell Microbiol. 2008 Oct;10(10):2078-90. doi: 10.1111/j.1462-5822.2008.01190.x. Epub 2008 Jul 10. Cell Microbiol. 2008. PMID: 18637021 Free PMC article. - Leishmania LABCG1 and LABCG2 transporters are involved in virulence and oxidative stress: functional linkage with autophagy.
Manzano JI, Perea A, León-Guerrero D, Campos-Salinas J, Piacenza L, Castanys S, Gamarro F. Manzano JI, et al. Parasit Vectors. 2017 May 30;10(1):267. doi: 10.1186/s13071-017-2198-1. Parasit Vectors. 2017. PMID: 28558770 Free PMC article. - Lipid hijacking: a unifying theme in vector-borne diseases.
O'Neal AJ, Butler LR, Rolandelli A, Gilk SD, Pedra JH. O'Neal AJ, et al. Elife. 2020 Oct 29;9:e61675. doi: 10.7554/eLife.61675. Elife. 2020. PMID: 33118933 Free PMC article. Review. - Synthetic glycovaccine protects against the bite of leishmania-infected sand flies.
Rogers ME, Sizova OV, Ferguson MA, Nikolaev AV, Bates PA. Rogers ME, et al. J Infect Dis. 2006 Aug 15;194(4):512-8. doi: 10.1086/505584. Epub 2006 Jul 3. J Infect Dis. 2006. PMID: 16845636 Free PMC article.
References
- Beverley, S. M. & Turco, S. J. (1998) Trends Microbiol. 6, 35-40. - PubMed
- Ilg, T. (2001) Med. Microbiol. Immunol. 190, 13-17. - PubMed
- Ilgoutz, S. C. & McConville, M. J. (2001) Int. J. Parasitol. 31, 899-908. - PubMed
- Turco, S. J., Späth, G. F. & Beverley, S. M. (2001) Trends Parasitol. 17, 223-226. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical