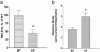Cathepsin L in secretory vesicles functions as a prohormone-processing enzyme for production of the enkephalin peptide neurotransmitter - PubMed (original) (raw)
. 2003 Aug 5;100(16):9590-5.
doi: 10.1073/pnas.1531542100. Epub 2003 Jul 17.
Doron Greenbaum, Katalin F Medzihradszky, Thomas Toneff, Richard Bundey, Ruthellen Miller, Birgit Schilling, Ivonne Petermann, Jessica Dehnert, Anna Logvinova, Paul Goldsmith, John M Neveu, William S Lane, Bradford Gibson, Thomas Reinheckel, Christoph Peters, Matthew Bogyo, Vivian Hook
Affiliations
- PMID: 12869695
- PMCID: PMC170962
- DOI: 10.1073/pnas.1531542100
Cathepsin L in secretory vesicles functions as a prohormone-processing enzyme for production of the enkephalin peptide neurotransmitter
Sukkid Yasothornsrikul et al. Proc Natl Acad Sci U S A. 2003.
Abstract
Multistep proteolytic mechanisms are essential for converting proprotein precursors into active peptide neurotransmitters and hormones. Cysteine proteases have been implicated in the processing of proenkephalin and other neuropeptide precursors. Although the papain family of cysteine proteases has been considered the primary proteases of the lysosomal degradation pathway, more recent studies indicate that functions of these enzymes are linked to specific biological processes. However, few protein substrates have been described for members of this family. We show here that secretory vesicle cathepsin L is the responsible cysteine protease of chromaffin granules for converting proenkephalin to the active enkephalin peptide neurotransmitter. The cysteine protease activity was identified as cathepsin L by affinity labeling with an activity-based probe for cysteine proteases followed by mass spectrometry for peptide sequencing. Production of [Met]enkephalin by cathepsin L occurred by proteolytic processing at dibasic and monobasic prohormone-processing sites. Cellular studies showed the colocalization of cathepsin L with [Met]enkephalin in secretory vesicles of neuroendocrine chromaffin cells by immunofluorescent confocal and immunoelectron microscopy. Functional localization of cathepsin L to the regulated secretory pathway was demonstrated by its cosecretion with [Met]enkephalin. Finally, in cathepsin L gene knockout mice, [Met]enkephalin levels in brain were reduced significantly; this occurred with an increase in the relative amounts of enkephalin precursor. These findings indicate a previously uncharacterized biological role for secretory vesicle cathepsin L in the production of [Met]enkephalin, an endogenous peptide neurotransmitter.
Figures
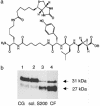
Fig. 1.
DCG-04 affinity labeling of the cysteine protease (PTP) from secretory vesicles. (a) Structure of DCG-04, an activity-based probe for cysteine proteases. The modified cysteine protease inhibitor DCG-04, resulting from biotinylation of E64-c, was used for affinity labeling. (b) Enrichment of the 27-kDa component by DCG-04 affinity labeling during purification of PE-cleaving activity. Relative PE-cleaving activities (PTP) in enriched fractions during purification were 1, 1, 26, and 90 relative units of activity at the steps of lysed chromaffin granules (CG), soluble CG extract (sol.), S200 gel filtration (S200), and chromatofocusing (lanes 1–4, respectively). Protein contents in lanes 1–4 were 170, 110, 2.5, and 1 μg, respectively.
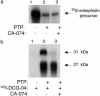
Fig. 2.
Specific affinity labeling with DCG-04, in the presence of CA-074, identifies the 27-kDa band as the enzyme for PE-cleaving activity. (a) CA-074 does not inhibit PE-cleaving activity. [35S]Enkephalin precursor was incubated with purified PTP in the absence (lane 2) or presence (lane 3) of CA-074 (1 μM) at 37°C for 2 h. Control [35S]enkephalin precursor incubated alone is shown (lane 1). Assays were assessed by SDS/PAGE and autoradiography. (b) DCG-04 affinity labeling of the 27-kDa component is not affected by CA-074. Affinity labeling of PTP by [125I]DCG-04 was conducted in the absence (lane 2) or presence of CA-074 (1 μM, lane 3). Control [125I]DCG-04 alone (no enzyme) was included (lane 1).
Fig. 3.
2D gel analyses of DCG-04 affinity-labeled PE-processing activity. (a) DCG-04-labeled PTP. DCG-04 labeling of purified PE-processing activity on 2D gels was blotted and visualized by avidin-horseradish peroxidase (HRP). (b) DCG-04-labeled enzymes detected on 2D gels. DCG-04-labeled enzyme proteins were subjected to 2D gels and silver staining.
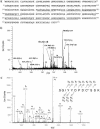
Fig. 4.
Bovine cathepsin L identified by MS. (a) Primary sequence of bovine cathepsin L. Peptides derived from tryptic digests of DCG-04 affinity-labeled 27-kDa proteins, sequenced by CID (tandem MS) MS, are illustrated as the underlined amino acid sequences of bovine cathepsin L. (b) Electrospray mass spectrum of the unfractionated tryptic digest of DCG-04 affinity-labeled 27-kDa enzyme. The electrospray mass spectrum of tryptic peptides of spot 2 from the 2D gel (from Fig. 3_a_) is illustrated, which were also observed from spots 1 and 3. Primary sequences of these peptides corresponded to bovine cathepsin L, underlined in a. Ions representing the same species (Na-, K- adducts included) are labeled with the same symbol. (c) Low-energy CID spectrum of tryptic peptide (residues 262–273). The tryptic peptide (residues 262–273) was observed from DCG-04-labeled spots 1–3. The CID spectrum for this precursor ion of m/z 703.27(2+) [nomenclature of Biemann (32)] indicated the peptide sequence Ser262-Gly-Ile-Tyr-Tyr-Asp-Pro-Asp-Cys(acrylamide)-Ser-Ser-Lys273, thus establishing Ser-272 instead of Cys-272, which have both been reported in GenBank. C*, acrylamide-modified cysteine.
Fig. 5.
Cathepsin L cleaves dibasic and monobasic processing sites of enkephalin-containing peptide substrates, BAM-22P, and Peptide F. BAM-22P (a) and Peptide F (b) were incubated with cathepsin L, and peptide products were identified by MALDI-TOF MS. The sequence of ME, YGGFM, is underlined. MS spectra and monoisotopic masses of peptide products are shown in Fig.8, which is published as supporting information on the PNAS web site,
. Cathepsin L cleavage sites are illustrated by solid arrows. Dotted-line arrows indicate secondary cleavage sites.
Fig. 6.
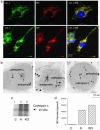
Localization of cathepsin L within enkephalin-containing secretory vesicles of neuroendocrine chromaffin cells. (a) Cathepsin L colocalization with ME by immunofluorescence confocal microscopy. Immunofluorescence localization of cathepsin L (cat. L) was assessed by anti-cathepsin L detected with anti-rabbit IgG-Alexa Fluor 488 (green fluorescence), and ME was detected with anti-ME and anti-mouse IgG-Alexa Fluor 594 (red). Colocalization is illustrated by overlay of the images, which is illustrated by yellow fluorescence. (b) Immunoelectron microscopy demonstrates colocalization of cathepsin L and ME in secretory vesicles. Cathepsin L in secretory vesicles was indicated by anti-cathepsin L detected with 15-nm colloidal gold-conjugated anti-rabbit IgG, and ME was detected with anti-ME and 6-nm colloidal gold-conjugated anti-mouse IgG. The presence of both 15- and 6-nm gold particles within these vesicles demonstrated the in vivo colocalization of cathepsin L and ME. (c) Stimulated secretion of cathepsin L from chromaffin cells. Secretion was induced with nicotine (10 μM) or KCl (50 mM) for 15 min, and [35S]cathepsin L in the secretion medium (with prior [35S]Met labeling) was detected by immunoprecipitation with anti-cathepsin L and SDS/PAGE. Secretion of [35S]cathepsin L was observed in nicotine-(N) and KCl-stimulated cells (lanes 2 and 3, respectively) but not in unstimulated control cells (C) (lane 1). (d) Stimulated secretion of ME from chromaffin cells. ME in the secretion medium from nicotine- or KCl-stimulated cells (for 15 min) (lanes 2 and 3, respectively) and unstimulated control cells (lane 1) were measured by an RIA. Values are averages of triplicate wells ± SEM.
Fig. 7.
Regulation of ME in cathepsin L gene KO mice. (a) ME (Met-Enk) levels in brains of KO mice. ME levels in extracts of brain tissue from cathepsin L gene KO (-/-) and WT control (+/+) mice were measured by an RIA, shown as the mean ± SEM, with 10 for each group. A significant decrease (*) in enkephalin levels in KO mice was observed (P < 0.013, two-tailed t test). (b) Ratio of precursor to enkephalin. The relative ratio of PE precursor to ME was determined by the ratio of ME measured (by an RIA) in tissue samples treated with trypsin and carboxypeptidase B compared with control untreated samples. A significant increase (*) in the ratio of precursor to ME in the KO mice was observed (P < 0.05, two-tailed t test).
Similar articles
- Evidence for functional localization of the proenkephalin-processing enzyme, prohormone thiol protease, to secretory vesicles of chromaffin cells.
Hook VY, Noctor S, Sei CA, Toneff T, Yasothornsrikul S, Kang YH. Hook VY, et al. Endocrinology. 1999 Aug;140(8):3744-54. doi: 10.1210/endo.140.8.6926. Endocrinology. 1999. PMID: 10433235 - Distinct properties of prohormone thiol protease (PTP) compared to cathepsins B, L, and H: evidence for PTP as a novel cysteine protease.
Azaryan AV, Hook VY. Azaryan AV, et al. Arch Biochem Biophys. 1994 Oct;314(1):171-7. doi: 10.1006/abbi.1994.1426. Arch Biochem Biophys. 1994. PMID: 7944391 - Secretory vesicle aminopeptidase B related to neuropeptide processing: molecular identification and subcellular localization to enkephalin- and NPY-containing chromaffin granules.
Hwang SR, O'Neill A, Bark S, Foulon T, Hook V. Hwang SR, et al. J Neurochem. 2007 Mar;100(5):1340-50. doi: 10.1111/j.1471-4159.2006.04325.x. Epub 2007 Jan 11. J Neurochem. 2007. PMID: 17241125 - Cathepsin L and Arg/Lys aminopeptidase: a distinct prohormone processing pathway for the biosynthesis of peptide neurotransmitters and hormones.
Hook V, Yasothornsrikul S, Greenbaum D, Medzihradszky KF, Troutner K, Toneff T, Bundey R, Logrinova A, Reinheckel T, Peters C, Bogyo M. Hook V, et al. Biol Chem. 2004 Jun;385(6):473-80. doi: 10.1515/BC.2004.055. Biol Chem. 2004. PMID: 15255178 Review. - Cysteine Cathepsins in the secretory vesicle produce active peptides: Cathepsin L generates peptide neurotransmitters and cathepsin B produces beta-amyloid of Alzheimer's disease.
Hook V, Funkelstein L, Wegrzyn J, Bark S, Kindy M, Hook G. Hook V, et al. Biochim Biophys Acta. 2012 Jan;1824(1):89-104. doi: 10.1016/j.bbapap.2011.08.015. Epub 2011 Sep 8. Biochim Biophys Acta. 2012. PMID: 21925292 Free PMC article. Review.
Cited by
- Human cathepsin V protease participates in production of enkephalin and NPY neuropeptide neurotransmitters.
Funkelstein L, Lu WD, Koch B, Mosier C, Toneff T, Taupenot L, O'Connor DT, Reinheckel T, Peters C, Hook V. Funkelstein L, et al. J Biol Chem. 2012 May 4;287(19):15232-41. doi: 10.1074/jbc.M111.310607. Epub 2012 Mar 5. J Biol Chem. 2012. PMID: 22393040 Free PMC article. - Reprint of: Catestatin: a multifunctional peptide from chromogranin A.
Mahata SK, Mahata M, Fung MM, O'Connor DT. Mahata SK, et al. Regul Pept. 2010 Nov 30;165(1):52-62. doi: 10.1016/j.regpep.2010.09.007. Epub 2010 Oct 20. Regul Pept. 2010. PMID: 20965217 Free PMC article. - A large form of secretogranin III functions as a sorting receptor for chromogranin A aggregates in PC12 cells.
Han L, Suda M, Tsuzuki K, Wang R, Ohe Y, Hirai H, Watanabe T, Takeuchi T, Hosaka M. Han L, et al. Mol Endocrinol. 2008 Aug;22(8):1935-49. doi: 10.1210/me.2008-0006. Epub 2008 May 15. Mol Endocrinol. 2008. PMID: 18483175 Free PMC article. - Novel odors affect gene expression for cytokines and proteinases in the rat amygdala and hippocampus.
Irwin LN, Byers DM. Irwin LN, et al. Brain Res. 2012 Dec 13;1489:1-7. doi: 10.1016/j.brainres.2012.10.034. Epub 2012 Oct 26. Brain Res. 2012. PMID: 23103411 Free PMC article. - Potential role of cytotoxic T-lymphocyte antigen 2 alpha in secretory activity of endocrine cells in mouse adenohypophysis.
Luziga C. Luziga C. Open Vet J. 2019 Jul;9(2):114-119. doi: 10.4314/ovj.v9i2.4. Epub 2019 Apr 12. Open Vet J. 2019. PMID: 31360649 Free PMC article.
References
- Gainer, H., Russell, J. T. & Loh, Y. P. (1985) Neuroendocrinology 40, 171-184. - PubMed
- Hook, V. Y. H., Azaryan, A. V. & Hwang, S. R. (1994) FASEB J. 8, 1269-1278. - PubMed
- Zhou, A., Webb, G., Zhu, X. R. & Steiner, D. F. (1999) J. Biol. Chem. 274, 20745-20748. - PubMed
- Seidah, N. G. & Prat, A. (2002) Essays Biochem. 38, 79-94. - PubMed
- Birch, N. P., Davie, A. D. & Christie, D. L. (1987) J. Biol. Chem. 262, 3382-3387. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous