The role of Six1 in mammalian auditory system development - PubMed (original) (raw)
The role of Six1 in mammalian auditory system development
Weiming Zheng et al. Development. 2003 Sep.
Abstract
The homeobox Six genes, homologues to Drosophila sine oculis (so) gene, are expressed in multiple organs during mammalian development. However, their roles during auditory system development have not been studied. We report that Six1 is required for mouse auditory system development. During inner ear development, Six1 expression was first detected in the ventral region of the otic pit and later is restricted to the middle and ventral otic vesicle within which, respectively, the vestibular and auditory epithelia form. By contrast, Six1 expression is excluded from the dorsal otic vesicle within which the semicircular canals form. Six1 is also expressed in the vestibuloacoustic ganglion. At E15.5, Six1 is expressed in all sensory epithelia of the inner ear. Using recently generated Six1 mutant mice, we found that all Six1(+/-) mice showed some degree of hearing loss because of a failure of sound transmission in the middle ear. By contrast, Six1(-/-) mice displayed malformations of the auditory system involving the outer, middle and inner ears. The inner ear development in Six1(-/-) embryos arrested at the otic vesicle stage and all components of the inner ear failed to form due to increased cell death and reduced cell proliferation in the otic epithelium. Because we previously reported that Six1 expression in the otic vesicle is Eya1 dependent, we first clarified that Eya1 expression was unaffected in Six1(-/-) otic vesicle, further demonstrating that the Drosophila Eya-Six regulatory cassette is evolutionarily conserved during mammalian inner ear development. We also analyzed several other otic markers and found that the expression of Pax2 and Pax8 was unaffected in Six1(-/-) otic vesicle. By contrast, Six1 is required for the activation of Fgf3 expression and the maintenance of Fgf10 and Bmp4 expression in the otic vesicle. Furthermore, loss of Six1 function alters the expression pattern of Nkx5.1 and Gata3, indicating that Six1 is required for regional specification of the otic vesicle. Finally, our data suggest that the interaction between Eya1 and Six1 is crucial for the morphogenesis of the cochlea and the posterior ampulla during inner ear development. These analyses establish a role for Six1 in early growth and patterning of the otic vesicle.
Figures
Fig. 1
Six1 expression during inner ear development. E8.75 to 15.5 _Six1lacZ_heterozygous embryos or inner ears were stained with X-gal for _Six1_lacZ and sectioned through the inner ear region. (A) A transverse section showing Six1 expression in the otic pit (op) at E8.75. (B) A transverse section showing Six1 expression in the otic cup (oc) at E9.0. (C) A transverse section showing Six1 expression in the middle and ventral otic vesicle (ov) at E9.5. Note Six1 is excluded from the dorsal otic pit and vesicle (arrows). (D) A transverse section at E12.5 showing Six1 expression in the saccular region and the vestibuloacoustic ganglion (gVIII). For A-D, dorsal is upwards. (E) A section showing Six1 expression in the primordia of lateral (la) and anterior (aa) Crista ampullaris. Arrow indicates the origin of the anterior ampulla on the other side. (F) A section showing Six1 expression in the hair cells (hc) of the utricle (u). (G) In the cochlea, Six1 is expressed throughout the future greater and lesser epithelial ridge (GER and LER). Note its expression level is reduced in an area that will become the organ of Corti (bracket) at E12.5. (H) In E15.5 cochlea, Six1 is expressed weakly in the supporting cells and first appeared in the inner hair cell (arrowhead) in the apex. Strong Six1 expression was also observed in the cells flanking the developing organ of Corti in the GER and LER. (I) In the base of E15.5 cochlea, Six1 is expressed in the outer (arrow) and inner (arrowhead) hair cells. It is also expressed in some cells in the GER. In addition, it is expressed in the thinner part of the cochlea duct that will probably differentiate into the stria vascularis (sv). (J) A latex paintfilled E15.5 cochlea showing the apical and basal regions. Scale bars: 50 µm.
Fig. 2
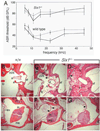
ABR threshold measurements and pathological structures in Six1+/− adult mouse ears. (A) Average threshold±s.e.m. for wild-type and Six1+/− ears in backgrounds 129/Sv and C57BL/6J (129/Sv, _n_=6 wild type, 13 Six1+/−; C57BL/6J, _n_=6 wild type, 10 Six1+/−). Averages used 90 dB SPL for thresholds beyond the upper limit of the sound system. The hearing loss was variable among mice and ears. Of the 13 Six1+/− mice tested, eight mice had severe hearing loss in both ears (threshold shifted by 50 dB between 15 and 32 kHz), two mice had mild hearing loss in both ears (threshold shifted by 20 dB), whereas three mice had normal hearing in the right ears and >70 dB loss in the left ears. Broken lines, C57BL/6J strain; unbroken lines, 129/Sv strain. (B-G) Histological analysis in wild type and Six1+/− ears. (B) Wild-type ear showing the stapes seated in the oval window (not visible because apposed by the stapes footplate, sf), and one arch of the stapes (s). The stapedial artery (a) is normally positioned. Part of the long process of the malleus (lp) is also present in this section. nVII, the VIIth cranial nerve. (C,D) Six1+/− ears showing that the footplates of the stapes are seated in the oval window. However, the VIIth nerve passed abnormally close to the oval window and the stapes or filled up the middle ear space near the oval window (arrows). Part of the stapes arches and the long process of the malleus are present in these sections. (E) Wild-type superior region of the middle ear space showing the junction of the malleus (ma) and incus (in). tm, tympanic membrane. (F,G) Six1+/− superior region of the middle ears showing the junction of the malleus and incus. The middle ear space was small and partially filled with loose connective tissue (arrow). The tympanic membrane was also abnormal (arrowheads). ABR threshold from these ears demonstrated a severe hearing loss. Scale bars: 100 µm.
Fig. 3
Auditory system development in Six1 homozygotes. (A,B) All Six1 homozygotes die at birth and exhibit severe auditory system defects involving the outer (arrow), middle and inner ears, as well as other defects. (C,D) Microdissected middle ear ossicles from E18.5 wild-type and _Six1_−/− embryos. In the mutant, the incus (in) is malformed and fused with the malleus (ma) (arrowheads) and the stapes (st) is absent. The short process (sp) of the malleus is often missing (arrow) and the long process (lp) is also shortened. (E,F) Transverse sections of E10.5 Six1 heterozygous and homozygous embryos stained with Hematoxylin and Eosin showing the developing otic vesicle (ov) and the vestibuloacoustic ganglion (gVIII). In Six1−/− embryos, although the otic vesicle formed, it appeared much smaller and abnormal (arrow) and the gVIII is absent (arrowhead). (G,H) Transverse sections of E12.5 wild-type and Six1 mutant embryos stained with X-gal for _Six1_lacZ and counterstained with diluted Hematoxylin showing the developing inner ear, _Six1lacZ_expression in the utricle and saccule region, semicircular canals, cranial ganglia gIX, gVIII and gV in the heterozygotes. However, in _Six1_−/− embryos, only malformed semicircular canal-like structure was observed (arrow). Other inner ear structures are not formed and gIX (arrowhead) and gVIII (asterisk) are absent in the homozygotes. (I,J) TUNEL analysis of transverse sections through the ear region of Six1+/− and _Six1_−/− embryos at E9.5. Numerous apoptotic cells are only detected in the lateral wall of _Six1_−/− otic vesicle (arrow). Scale bars: 100 µm.
Fig. 4
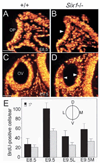
Six1 controls proliferation of otic epithelial cells during early inner ear development. (A-D) Transverse sections of otic regions from E8.5 (A,B) and E9.5 (C,D) wild-type and _Six1_−/− embryos showing BrdU-labeled cells (orange). BrdU-positive cells were slightly reduced in _Six1_−/− otic placode (OP) at E8.5 (B) and by E9.5, BrdU-positive cells were largely reduced in the dorsal region of _Six1_−/− otic vesicle (OV, above arrowheads in D). (E) The labeling index was determined by counting the number of BrdU-positive cells from each otic placode or vesicle. Ten ears for each genotype were counted and the numbers were averaged. At E8.5, the number of BrdU-labeled cells in _Six1_−/− otic placode was 80% of wild-type embryos. By E9.5, the number of BrdU-labeled cells in _Six1_−/− otic vesicle was reduced to 50% of that in wild-type embryos. In _Six1_−/− embryos, the number of BrdU-positive cells in the lateral otic vesicle was reduced to 60% (E9.5L), while in the medial half the number was reduced to 40% of that in wild-type embryos (E9.5M). Scale bars: 50 µm.
Fig. 5
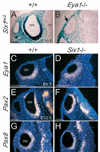
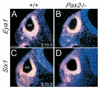
Eya1, Pax2 and Pax8 expression in otic epithelium is _Six1_-independent. (A,B) Six1lacZ is normally expressed in otic vesicle (ov) and its expression was undetectable in _Eya1_−/− embryos. (C-H) Eya1 (C,D), Pax2 (E,F) and Pax8 (G,H) expression levels in otic vesicle were unaffected in _Six1_−/− embryos. Scale bars: 100 µm. nt, neural tube.
Fig. 6
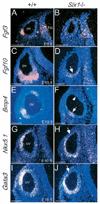
Loss of Six1 function alters the expression of Fgf3, Fgf10, Bmp4, Nkx5.1 and Gata3 in the otic vesicle. (A-D,G-J) Transverse sections; (E,F) Horizontal sections. (A,B) Fgf3 begins to be expressed in the ventrolateral region of the otic vesicle (ov) at E9.5 and its expression was undetectable in _Six1_−/− embryos. Note Fgf3 expression in the hindbrain (hb) is relatively normal in _Six1_−/− embryos. (C,D) Fgf10 is normally expressed in a broad region of ventral otic vesicle and the gVIII in wild-type embryos at E10.5; however, residual expression of Fgf10 was only detected in the ventromedial otic vesicle in _Six1_−/− embryos (arrow). (E,F) Bmp4 is normally observed in two specific spots, one dorsal and one ventrolateral in the otic vesicle of wild-type embryos at E10.5. In _Six1_−/− embryos, the dorsal expression spot (arrowhead) was undetectable and the ventrolateral spot became very weak (arrow). (G,H) Nkx5.1 is expressed in the dorsolateral region of the otic vesicle in wild-type embryos at E10.5. In _Six1_−/− embryos, however, Nkx5.1 expression is excluded from the dorsolateral region (arrow) and its expression domain shifted or expanded ventrally, including the ventral-most region. (I,J) Gata3 is expressed strongly dorsolaterally and weakly ventromedially in the wild-type embryos at E10.5. Similarly, in _Six1_−/− embryos it is not expressed in the dorsolateral region (arrow) and its expression domain shifted ventrally. By contrast, its expression in the ventromedial region of the otic vesicle was unaffected in _Six1_−/− embryos. Scale bars: 100 µm.
Fig. 7
Six1 and Eya1 expression in the otic vesicle is Pax2 independent. (A-D) Eya1 (A,B) and Six1 (C,D) are expressed in the ventral otic vesicle (ov) and its derivative gVIII of wild-type embryos at E10.5 and their expression was unaffected in these structures in _Pax2_−/− embryos. Scale bars: 100 µm.
Fig. 8
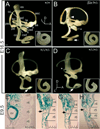
Enhancement of inner ear defects in Eya1/Six1 double mutants. (A-D) Medial (A-C) and lateral (D) views of paintfilled inner ears of wild-type (A), Six1+/− (B), and Six1+/−_/Eya1_+/− compound heterozygous (C,D) embryos at E16.5. (A) All structures of the inner ear reached their mature shape at E16.5. The cochlea completed 1.75 turns by this stage (inset). aa, anterior ampulla; asc, anterior semicircular canal; cc, common crus; co, cochlea; csd, cochleosaccular duct; ed, endolymphatic duct; es, endolymphatic sac; la, lateral ampulla; lsc, lateral semicircular canal; pa, posterior ampulla; psc, posterior semicircular canal; s, saccule; u, utricle; usd, utriculosaccular duct. (B) A Six1+/− inner ear showing a malformed saccule (asterisk), absence of the endolymphatic sac and a truncated endolymphatic duct (arrowhead). The cochlea only completed 1.5 turns (arrow and inset). (C,D) Inner ears from Six1+/−/Eya1+/− double heterozygotes showing severe cochlear defects (arrows), absence of posterior ampulla (asterisk) and truncated posterior semicircular canal. The cochlea only completed less than one turn and their distal tips were enlarged and mal-shaped (insets). (E-H) Frontal histological sections at comparable levels of X-gal-stained E9.5 embryos of Eya1/Six1 double heterozygous (E) and double homozygous embryos (F-H). Eya1/Six1 double heterozygote shows restricted _Six1lacZ_expression in the otic vesicle (ov). In the double homozygotes, the otic vesicles appeared to be formed in the correct position but severely hypoplastic. Because Six1lacZ is Eya1 dependent, it is not expressed in _Eya1_−/−/_Six1_−/− otic vesicle (arrows) but is ectopically turned on in rhombomeres 2, 4 and 6 (r4 and r6). Scale bars: 100 µm.
Similar articles
- Eya1 regulates the growth of otic epithelium and interacts with Pax2 during the development of all sensory areas in the inner ear.
Zou D, Silvius D, Rodrigo-Blomqvist S, Enerbäck S, Xu PX. Zou D, et al. Dev Biol. 2006 Oct 15;298(2):430-41. doi: 10.1016/j.ydbio.2006.06.049. Epub 2006 Jul 7. Dev Biol. 2006. PMID: 16916509 Free PMC article. - Eya1-deficient mice lack ears and kidneys and show abnormal apoptosis of organ primordia.
Xu PX, Adams J, Peters H, Brown MC, Heaney S, Maas R. Xu PX, et al. Nat Genet. 1999 Sep;23(1):113-7. doi: 10.1038/12722. Nat Genet. 1999. PMID: 10471511 - Six1 controls patterning of the mouse otic vesicle.
Ozaki H, Nakamura K, Funahashi J, Ikeda K, Yamada G, Tokano H, Okamura HO, Kitamura K, Muto S, Kotaki H, Sudo K, Horai R, Iwakura Y, Kawakami K. Ozaki H, et al. Development. 2004 Feb;131(3):551-62. doi: 10.1242/dev.00943. Epub 2003 Dec 24. Development. 2004. PMID: 14695375 - The development of the vertebrate inner ear.
Torres M, Giráldez F. Torres M, et al. Mech Dev. 1998 Feb;71(1-2):5-21. doi: 10.1016/s0925-4773(97)00155-x. Mech Dev. 1998. PMID: 9507049 Review. - A symphony of inner ear developmental control genes.
Chatterjee S, Kraus P, Lufkin T. Chatterjee S, et al. BMC Genet. 2010 Jul 16;11:68. doi: 10.1186/1471-2156-11-68. BMC Genet. 2010. PMID: 20637105 Free PMC article. Review.
Cited by
- The Eya1 phosphatase promotes Shh signaling during hindbrain development and oncogenesis.
Eisner A, Pazyra-Murphy MF, Durresi E, Zhou P, Zhao X, Chadwick EC, Xu PX, Hillman RT, Scott MP, Greenberg ME, Segal RA. Eisner A, et al. Dev Cell. 2015 Apr 6;33(1):22-35. doi: 10.1016/j.devcel.2015.01.033. Epub 2015 Mar 26. Dev Cell. 2015. PMID: 25816987 Free PMC article. - EYA1-SIX1 complex in neurosensory cell fate induction in the mammalian inner ear.
Wong EY, Ahmed M, Xu PX. Wong EY, et al. Hear Res. 2013 Mar;297:13-9. doi: 10.1016/j.heares.2012.09.009. Epub 2012 Oct 24. Hear Res. 2013. PMID: 23104013 Free PMC article. Review. - A Novel ENU-Induced Mutation in Myo6 Causes Vestibular Dysfunction and Deafness.
Wong EY, Xu CY, Brahmachary M, Xu PX. Wong EY, et al. PLoS One. 2016 May 12;11(5):e0154984. doi: 10.1371/journal.pone.0154984. eCollection 2016. PLoS One. 2016. PMID: 27171474 Free PMC article. - Six1 proteins with human branchio-oto-renal mutations differentially affect cranial gene expression and otic development.
Shah AM, Krohn P, Baxi AB, Tavares ALP, Sullivan CH, Chillakuru YR, Majumdar HD, Neilson KM, Moody SA. Shah AM, et al. Dis Model Mech. 2020 Mar 3;13(3):dmm043489. doi: 10.1242/dmm.043489. Dis Model Mech. 2020. PMID: 31980437 Free PMC article. - Six1 expression is associated with a poor prognosis in patients with glioma.
Zhang X, Xu R. Zhang X, et al. Oncol Lett. 2017 Mar;13(3):1293-1298. doi: 10.3892/ol.2017.5577. Epub 2017 Jan 10. Oncol Lett. 2017. PMID: 28454249 Free PMC article.
References
- Buller C, Xu X, Marquis V, Schwanke R, Xu P-X. Molecular effects of Eya1 domain mutations causing organ defects in BOR syndrome. Hum. Mol. Genet. 2001;10:2775–2781. - PubMed
- Burk DT, Willhite CC. Inner ear malformations induced by isotretinoin in hamster fetuses. Teratology. 1992;46:147–157. - PubMed
- Chang W, Nunes FD, de Jesus-Escobar JM, Harland R, Wu DK. Ectopic noggin blocks sensory and nonsensory organ morphogenesis in the chicken inner ear. Dev. Biol. 1999;216:369–381. - PubMed
- Chen R, Amoui M, Zhang Z, Mardon G. Dachshund and eyes absent proteins form a complex and function synergistically to induce ectopic eye formation in Drosophila. Cell. 1997;91:893–903. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P20 RR015583/RR/NCRR NIH HHS/United States
- R01 DC005824/DC/NIDCD NIH HHS/United States
- P20 RR 15583/RR/NCRR NIH HHS/United States
- R01 DC 05824/DC/NIDCD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials