Co-expression of p16(INK4A) and laminin 5 gamma2 by microinvasive and superficial squamous cell carcinomas in vivo and by migrating wound and senescent keratinocytes in culture - PubMed (original) (raw)
Co-expression of p16(INK4A) and laminin 5 gamma2 by microinvasive and superficial squamous cell carcinomas in vivo and by migrating wound and senescent keratinocytes in culture
Easwar Natarajan et al. Am J Pathol. 2003 Aug.
Abstract
The high frequency of mutation, deletion, and promoter silencing of the gene encoding p16(INK4A) (p16) in premalignant dysplasias and squamous cell carcinomas (SCC) of epidermis and oral epithelium classifies p16 as a tumor suppressor. However, the point during neoplastic progression at which this protein is expressed and presumably impedes formation of an SCC is unknown. Induction of p16 has been found to be responsible for the senescence arrest of normal human keratinocytes in culture, suggesting the possibility that excessive or spatially abnormal cell growth in vivo triggers p16 expression. We examined 73 skin and oral mucosal biopsy specimens immunohistochemically to test this hypothesis. p16 was not detectable in benign hyperplastic lesions, but instead was expressed heterogeneously in some dysplastic and carcinoma in situ lesions and consistently at areas of microinvasion and at superficial margins of advanced SCCs. p16-positive cells in these regions coexpressed the gamma2 chain of laminin 5, identified previously as a marker of invasion in some carcinomas. Normal keratinocytes undergoing senescence arrest in culture proved to coordinately express p16 and gamma2 and this was frequently associated with increased directional motility. Keratinocytes at the edges of wounds made in confluent early passage cultures also coexpressed p16 and gamma2, accompanying migration to fill the wound. These results have identified the point during neoplastic progression in stratified squamous epithelial at which the tumor suppressor p16 is expressed and suggest that normal epithelia may use the same mechanism to generate non-dividing, motile cells for wound repair.
Figures
Figure 1.
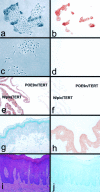
Optimization of the p16 immunostaining protocol. a and b: Phase contrast (a) and brightfield (b) views of normal primary epidermal keratinocyte strain N cells stained for p16 (brown). Note that small, dividing cells are p16-negative and large, senescence-arrested cells are p16-positive, with predominantly cytoplasmic staining, as previously described. c and d: Phase contrast (c) and brightfield (d) views of epidermal SCC line SCC-13 cells stained for p16. Note absence of p16 staining. e and f: p16 immunostaining (brown) of formalin-fixed, paraffin-embedded cultured cells of the p16-expressing line N/p53DD/cdk4R/TERT (N/P/C/TERT) and the p16 gene-deleted line POE-9n/TERT, following baking of sections onto slides for 30 minutes at 37°C (e) or at 55°C (f). Note that baking at 37°C yielded accurate p16 staining of these lines, while baking at 55°C resulted in virtually no p16 staining of N/p53DD/cdk4R/TERT. g and h: p16 immunostaining (brown) of human foreskin specimen fixed in 10% formalin for 1 day (g) or 3 days (h) before embedding. Section shown in g was counterstained with hematoxylin. Note accurate absence of p16 staining of epidermis fixed for 1 day and artifactual homogenous staining of all living layers of the epidermis after fixation for 3 days. i and j: Benign papillary hyperplasia and hyperkeratosis of alveolar ridge epithelium stained with H&E (i) or for p16 (j). Section shown in j was counterstained with hematoxylin. Note absence of p16 staining.
Figure 2.
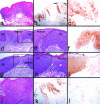
p16 expression in epidermal dysplasia and CIS. a to c: Seborrheic keratosis, exhibiting hyperkeratosis and benign hyperplasia. a and b: H&E. b: p16 immunostain (brown). b: Magnification of area marked in a. c: Magnification of area marked in b. Note weak, homogenous staining present throughout the epithelium (dark spots in some cells in the basal layer are melanin in melanocytes) and in cells of the underlying dermis, interpretable as non-specific background staining caused by prolonged formalin fixation of the specimen. d–f: Dysplastic epithelium with budding into connective tissue, adjacent to invasive SCC (not shown in field). d: H&E. e and f: p16 immunostain (brown). Note heterogeneous, cytoplasmic p16 staining of CIS. g–i: CIS adjacent to normal-appearing epithelium. g: H&E. (h and i) p16 immunostain (brown). i: Magnification of area marked in h. Note variegated p16 staining of CIS. j and k: SCC with overlying dysplastic/atypical epithelium. j: H&E. k: p16 immunostain (brown). k: Magnification of area marked in j. Note that the SCC is p16-negative, while the overlying dysplastic/atypical epithelium exhibits heterogeneous p16 staining.
Figure 3.
p16 expression in oral dysplasia, CIS, and superficially invasive SCC. a–c, d–f, g–i, and j–l: Four pathological epithelial specimens stained with H&E (a,d,e,g,h,j) and for p16 (b,c,f,i,k,l). a–c: Moderate dysplasia. c: Magnification of the area demarcated in b. Note variegated p16 staining patterns. d–f and g–i: Two cases of CIS adjacent to normal-appearing epithelium. e and h: Magnifications of the areas demarcated in d and g, respectively. Lines in e and h show the border between histologically normal epithelium and CIS. Note variegated p16 staining in CIS areas in f and homogenous p16 expression in CIS areas in i, with absence of p16 in intervening areas of normal-appearing epithelium. Specimen (g–i) was found by PCR analysis to contain HPV31 DNA (see Results). j–l: Advanced SCC. The upper left corner of j shows the external surface of the tumor and the arrow indicates the point below which islands of SCC cells are growing beneath the normal thickness of the mucosal connective tissue. l: A region of the specimen to the lower right and outside the area shown in j. Note variegated p16 staining in superficial regions and paucity of p16-positive cells in deeper regions of the tumor.
Figure 4.
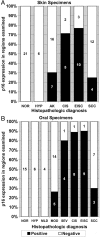
Incidence of p16-positive cells in skin and oral tissue specimens exhibiting various degrees of neoplasia. A: Skin specimens. B: Oral specimens. As described in Results, staining was evaluated for histologically distinct areas within 36 skin and 37 oral specimens, yielding a total of 85 areas in skin specimens and 77 in oral specimens. Numbers on bars indicate the number of areas having the indicated p16 phenotype. Note that normal and benign hyperplastic epithelium was p16-negative, while a large majority of advanced dysplasias and CIS lesions contained p16-positive cells. Microinvasive regions and superficially invading regions at the lateral margins of SCCs were also p16-positive, while deeper regions of SCCs were mostly p16-negative with rare p16-positive cells located primarily in terminally differentiated whorls.
Figure 5.
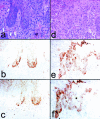
p16 and laminin 5γ2 coexpression in dysplasias and microinvasive SCC. a and d: H&E. b and e: p16 staining. c and f: γ2 staining. a–c: Area of mild-to-moderate dysplasia in oral epithelium adjacent to invasive SCC (outside the area shown). d–f: superficially invasive SCC. Note variegated p16 and γ2 co-expression in epithelial cells at the connective tissue interface at sites of incipient or early invasion.
Figure 6.
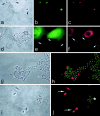
Coexpression of p16 and laminin 5γ2 by senescence-arrested cells in cultures of primary human keratinocytes. Four areas (a–c, d–f, g and h, and i and j) in cultures of strain N keratinocytes. a: Phase contrast. b: p16. c: γ2. Note coexpression of p16 and γ2 by three large cells. d: Phase contrast. e: p16 staining. f: γ2 staining of magnified area in d. Note coexpression of p16 and high levels of γ2 by large cells with adjacent smaller cells (arrows in e and f) being p16-negative and with low levels of γ2. g: Phase contrast. h: Double immunostaining for p16 (red) and BUdR (green). Note inverse correlation between cell division and p16 expression, as previously described. i: Phase contrast. j: Double immunostaining for γ2 (red) and BUdR (green). Note inverse correlation between cell division and γ2 expression, with small, dividing keratinocytes shown with asterisk and large, growth-arrested keratinocytes shown by arrows.
Figure 7.
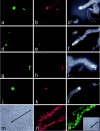
Coexpression of p16 and laminin 5γ2 by keratinocytes in culture; correlation with directional motility. Panels show OKB8 (a–c), strain N (d–f, j–l, m–o), and N/TERT-2G (g–i) keratinocyte cultures immunostained for p16 (a,d,g,j,o) or γ2 (b,c,e,f,h,i,k,l,n). m: Phase contrast view of the field shown in n. Inset in o: Phase contrast view of the field shown in o. Panels c, f, i, and l are overexposed, grayscale versions of b, e, h, and k, respectively, revealing the migration history of cells by their deposition of γ2 on the culture dish surface. Note coexpression of p16 and γ2 and substantial directional motility of single p16/γ2-positive cells and small colonies that contain one or more p16/γ2-positive cells. Note up-regulation of γ2 and p16 by the cells at the edge of experimental wounds made in a confluent monolayer of early passage keratinocytes.
References
- Bouquot JE, Weiland LH, Kurland LT: Leukoplakia and carcinoma in situ synchronously associated with invasive oral/oropharyngeal carcinoma in Rochester, Minn, 1935–1984. Oral Surg Oral Med Oral Pathol 1988, 65:199-207 - PubMed
- Mashberg A, Feldman LJ: Clinical criteria for identifying early oral and oropharyngeal carcinoma: erythroplasia revisited. Am J Surg 1988, 156:273-275 - PubMed
- Lee JJ, Hong WK, Hittelman WN, Mao L, Lotan R, Shin DM, Benner SE, Xu XC, Lee JS, Papadimitrakopoulou VM, Geyer C, Perez C, Martin JW, El-Naggar AK, Lippman SM: Predicting cancer development in oral leukoplakia: ten years of translational research. Clin Cancer Res 2000, 6:1702-1710 - PubMed
- Kamb A, Gruis NA, Weaver-Feldhaus J, Liu Q, Harshman K, Tavtigian SV, Stockert E, Day RS, III, Johnson BE, Skolnick MH: A cell cycle regulator potentially involved in genesis of mamny tumor types. Science 1994, 264:436-440 - PubMed
- Yeudall WA, Crawford RY, Ensley JF, Robbins KC: MTS1/CDK4I is altered in cell lines derived from primary and metastatic oral squamous cell carcinoma. Carcinogenesis 1994, 15:2683-2686 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 AR042689/AR/NIAMS NIH HHS/United States
- R01 DE013178/DE/NIDCR NIH HHS/United States
- P30-AR42689/AR/NIAMS NIH HHS/United States
- R01-DE13178/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials