Transgenic overexpression of platelet-derived growth factor-C in the mouse heart induces cardiac fibrosis, hypertrophy, and dilated cardiomyopathy - PubMed (original) (raw)
Transgenic overexpression of platelet-derived growth factor-C in the mouse heart induces cardiac fibrosis, hypertrophy, and dilated cardiomyopathy
Annica Pontén et al. Am J Pathol. 2003 Aug.
Abstract
The platelet-derived growth factors are implicated in development of fibrotic reactions and disease in several organs. We have overexpressed platelet-derived growth factor-C in the heart using the alpha-myosin heavy chain promoter and created a transgenic mouse that exhibits cardiac fibrosis followed by hypertrophy with sex-dependent phenotypes. The transgenic mice developed several pathological changes including cardiac fibroblast proliferation and deposition of collagen, hypertrophy, vascular defects, and the presence of Anitschkow cells in the adult myocardium. Male mice developed a hypertrophic phenotype, whereas female mice were more severely affected and developed dilated cardiomyopathy, leading to heart failure and sudden death. The vascular defects initially included dilation of microvessels and vascular leakage. Subsequently, a marked loss of microvessels, formation of large vascular sac-like structures, and an increased density of smooth muscle-coated vessels were observed in the myocardium. In part, the observed vascular changes may be because of an up-regulation of vascular endothelial growth factor in cardiac fibroblasts of the transgenic hearts. This unique animal model reveals that a potent mitogen for cardiac fibroblasts result in an expansion of the interstitium that induce a secondary sex-dependent hypertrophic response in the cardiomyocytes.
Figures
Figure 1.
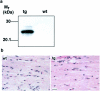
Transgenic expression of PDGF-C protein and gross phenotype of transgenic hearts. a: SDS-PAGE and immunoblot analysis of heart tissue lysates under reducing conditions. Expression of PDGF-C was detected in transgenic hearts (tg) at 1 month of age and the expression was persistent in hearts from 3-month-old animals. Both full-length PDGF-C (45 kd, top arrow) and processed species of ∼35 kd (bottom arrow) are visualized (top). The expression level and processing of transgenic PDGF-C were similar in females and males. Calnexin served as a loading control (bottom). b: Immunohistochemical staining of heart sections from 1-month-old mice. In wild-type hearts (wt) no expression was detected, whereas in the transgenic hearts (tg) PDGF-C was detected in the cytoplasm of a subset of cardiomyocytes at 1 month. Older mice expressed the transgene in the majority of cardiomyocytes. Scale, 20 μm. c: PDGFR-α is activated in PDGF-C transgenic mice. Heart lysates from 2-month-old transgenic animals were subjected to immunoprecipitation using a rabbit antiserum to PDGFR-α and the precipitates were analyzed by SDS-PAGE and immunoblotted using a monoclonal anti-phosphotyrosine antibody (top), or using an antiserum to the C-terminal of PDGFRs (bottom). Activated PDGFR-α is seen only in the transgenic heart (arrow, top). Mature (170 kd), and not fully processed (140 kd) PDGFR-α were found in the transgenic heart, whereas only mature (170 kd) PDGFR-α was found in wild-type hearts (arrows, bottom). d: RNase protection assay on total RNA isolated from transgenic (tg) and wild-type (wt) hearts using a PDGFR-α-specific riboprobe. Five transgenic and three wild-type animals were analyzed. PDGFR-α mRNA was up-regulated approximately threefold in transgenic hearts. A β-actin riboprobe was used as an internal control. e: PDGF-C transgenic mice have increased heart:body weight ratios. Comparison between mouse heart and body weights in wild-type (striped and checked bars) and transgenic animals (black and gray bars) of different ages (n = 4 to 8 animals in each group). The heart:body weight ratios in female transgenics (black bars) is dramatically increased compared to wild-type females (striped bars, P = 0.0013 for 1-month-old animals, and P = 0.013 for 3.5-month-old animals). Transgenic males (gray bars) develop a less abnormal ratio compared to the wild-type males (checked bars, P = 0.057 for 1-month-old animals, and P = 0.049 for 3.5-month-old animals). f: Cardiac hypertrophy in PDGF-C transgenic animals. Hearts from 3.5-month-old female wild-type (wt) and transgenic (tg) littermates are shown (top). Hearts from 6-month-old male wild-type (wt) and transgenic (tg) littermates (bottom). Scale, 5 mm. g: Fibroblasts proliferate in the transgenic heart. Immunohistochemical staining visualizing proliferating cell nuclear antigen in fibroblasts of transgenic (tg) heart at age 1 month compared with the wild-type (wt).
Figure 2.
Histology of PDGF-C transgenic hearts. a to g: H&E stainings on tissue sections from the left ventricle of the heart from a: a 1-month-old wild-type female; b: a 1-month-old transgenic male showing proliferation of interstitial fibroblasts; c: a 3-month-old wild-type female, d: a 3-month-old transgenic female; e: a 6-month-old wild-type male; f: a 6-month-old transgenic male with enlarged myocyte nuclei; g: a transgenic female with heart failure exhibiting a disorganized heart tissue architecture because of extensive interstitial fibrosis. h: Comparisons of ventricle size and wall thickness on H&E-stained heart tissue sections from males and females. Top: A comparison between 3.5-month-old wild-type (wt) and transgenic (tg) males. Middle: A comparison between 3.5-month-old wild-type (wt) and transgenic (tg) females. Bottom: A comparison of heart cross-sections from 6-month-old wild-type (wt) and transgenic (tg) males. In the transgenic female the size of ventricles is enlarged and ventricle walls are dilated. The transgenic males develop a different phenotype with a thickening of left ventricle wall leading to a smaller ventricle volume. Scale, 1 mm. i to n: Masson’s trichrome staining on heart tissue sections from the left ventricle. In the Masson’s trichrome staining, collagen is stained in blue; the nucleus in black; and cytoplasm, keratin, muscle fibers, and fibrin in red. The presence of collagenous extracellular matrix is indicated by the blue staining. Scale, 20 μm. i: Normal appearance of myocardium in 3-month-old wild-type female; j: 3-month-old transgenic male with thickened myofibers and deposition of extracellular matrix; k: 3-month-old wild-type female; l: 6-month-old wild-type male; m: a 6-month-old transgenic male showing extensive fibrosis and variable myofiber diameter; n: a section from a 5.5-month-old transgenic female that died from heart failure; o and p: H&E stainings of transgenic tissue sections showing pathological findings. Anitschkow cells with caterpillar-like chromatin patterns o: and presence of hyaline drops as evidence of hyaline degeneration p: Scale, 10 μm. q: Northern blot analysis on total heart RNA from 3- to 4-month-old wild-type (wt) (two females, two males) and transgenic (tg) (two females, two males) mice. Expression of ANP and BNP was highly up-regulated in the transgenic mice, whereas the expression of MLC-2 showed variations. The differences were more prominent in the females; ANP was up-regulated twofold to sixfold and BNP was up-regulated twofold to ninefold (compared to sex-matched wild types). MLC-2 was slightly down-regulated in females, whereas in the younger male MLC-2 was up-regulated twofold and in the older male MLC-2 was slightly down-regulated (compared to sex-matched wild types). The amount of RNA in each lane was normalized by analyzing the expression level of GAPDH.
Figure 3.
Transgenic mice with targeted overexpression of the CUB domain of PDGF-C in the heart a: SDS-PAGE and immunoblot analysis of heart tissue lysates under reducing conditions shows expression of 24-kd transgenic CUB. b: H&E stainings on tissue sections from the left ventricle of the heart from transgenic (tg) and wild-type (wt) littermates. Scale, 20 μm.
Figure 4.
Vascular changes in PDGF-C transgenic mice. Endothelial cell-specific staining using antibodies to PECAM (a–h), SMA-specific staining using antibodies to SMA (i, j), and VEGF-specific staining (k, l) of heart tissue sections from the left ventricle. a: A 3-month-old wild-type female showing a normal appearance of microvascular network. b and c: Variable degrees of vascular proliferation in a PDGF-C transgenic 3-month-old female. d and e: Wild-type female age 3 months compared (d) with transgenic male at lower magnification (e), showing the presence of larger vessels in the transgenic ventricle wall. f: Wild-type male age 6 months. g and h: Variable degrees of vascular proliferation in the same transgenic male at age 6 months. h: Clearly illustrated is a loss of capillaries in the transgenic heart. i: Normal distribution of smooth muscle-coated vessels in a 3-month-old wild-type female. j: Increased number of smooth muscle-coated vessels (arrows) and up-regulation of SMA in cardiomyocytes in a female transgenic littermate. Scale, 20 μm. k: VEGF staining of wild-type myocardium. l: VEGF staining of transgenic myocardial fibroblasts at 1 month of age. Scale, 20 μm.
Figure 5.
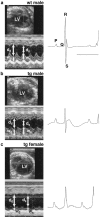
Representative transthoracic echocardiograms on wild-type and PDGF-C transgenic littermates at 3.5 months of age. End-diastolic left ventricle diameter (LV), and end-systolic and end-diastolic left ventricle wall thickness (ws and wd, respectively), and diameters (ds and dd, respectively) were measured (left). The corresponding electrocardiograms are shown at the right (bar, 100 ms). Fractional shortening, defined as (dd − ds)/dd, was impaired in the PDGF-C transgenic mice. a: A wild-type male with a normal appearance of the heart. b: A transgenic male with a thicker ventricle wall, a resulting smaller left ventricle volume, and decreased contractile function. c: A transgenic female with a dramatically enlarged left ventricle, a dilated ventricle wall, and an almost absent contractile function.
Similar articles
- Platelet-derived growth factor D induces cardiac fibrosis and proliferation of vascular smooth muscle cells in heart-specific transgenic mice.
Pontén A, Folestad EB, Pietras K, Eriksson U. Pontén A, et al. Circ Res. 2005 Nov 11;97(10):1036-45. doi: 10.1161/01.RES.0000190590.31545.d4. Epub 2005 Oct 13. Circ Res. 2005. PMID: 16224065 - Synthetic prostacyclin agonist, ONO1301, enhances endogenous myocardial repair in a hamster model of dilated cardiomyopathy: a promising regenerative therapy for the failing heart.
Ishimaru K, Miyagawa S, Fukushima S, Saito A, Sakai Y, Ueno T, Sawa Y. Ishimaru K, et al. J Thorac Cardiovasc Surg. 2013 Dec;146(6):1516-25. doi: 10.1016/j.jtcvs.2013.02.045. J Thorac Cardiovasc Surg. 2013. PMID: 24229503 - Neuron-derived orphan receptor-1 modulates cardiac gene expression and exacerbates angiotensin II-induced cardiac hypertrophy.
Cañes L, Martí-Pàmies I, Ballester-Servera C, Herraiz-Martínez A, Alonso J, Galán M, Nistal JF, Muniesa P, Osada J, Hove-Madsen L, Rodríguez C, Martínez-González J. Cañes L, et al. Clin Sci (Lond). 2020 Feb 14;134(3):359-377. doi: 10.1042/CS20191014. Clin Sci (Lond). 2020. PMID: 31985010 - Fibroblast-mediated pathways in cardiac hypertrophy.
Fujiu K, Nagai R. Fujiu K, et al. J Mol Cell Cardiol. 2014 May;70:64-73. doi: 10.1016/j.yjmcc.2014.01.013. Epub 2014 Jan 31. J Mol Cell Cardiol. 2014. PMID: 24492068 Review. - Various hypertrophic stimuli induce distinct phenotypes in cardiomyocytes.
Schaub MC, Hefti MA, Harder BA, Eppenberger HM. Schaub MC, et al. J Mol Med (Berl). 1997 Nov-Dec;75(11-12):901-20. doi: 10.1007/s001090050182. J Mol Med (Berl). 1997. PMID: 9428623 Review.
Cited by
- Cardiac progenitor cell cycling stimulated by pim-1 kinase.
Cottage CT, Bailey B, Fischer KM, Avitabile D, Collins B, Tuck S, Quijada P, Gude N, Alvarez R, Muraski J, Sussman MA. Cottage CT, et al. Circ Res. 2010 Mar 19;106(5):891-901. doi: 10.1161/CIRCRESAHA.109.208629. Epub 2010 Jan 14. Circ Res. 2010. PMID: 20075333 Free PMC article. - Inactivation of Smad5 in endothelial cells and smooth muscle cells demonstrates that Smad5 is required for cardiac homeostasis.
Umans L, Cox L, Tjwa M, Bito V, Vermeire L, Laperre K, Sipido K, Moons L, Huylebroeck D, Zwijsen A. Umans L, et al. Am J Pathol. 2007 May;170(5):1460-72. doi: 10.2353/ajpath.2007.060839. Am J Pathol. 2007. PMID: 17456754 Free PMC article. - Research Progress of Myocardial Fibrosis and Atrial Fibrillation.
Li G, Yang J, Zhang D, Wang X, Han J, Guo X. Li G, et al. Front Cardiovasc Med. 2022 Jul 25;9:889706. doi: 10.3389/fcvm.2022.889706. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35958428 Free PMC article. Review. - Transient receptor potential (TRP) channels and cardiac fibrosis.
Yue Z, Zhang Y, Xie J, Jiang J, Yue L. Yue Z, et al. Curr Top Med Chem. 2013;13(3):270-82. doi: 10.2174/1568026611313030005. Curr Top Med Chem. 2013. PMID: 23432060 Free PMC article. Review. - Expression and function of PDGF-C in development and stem cells.
Tian Y, Zhan Y, Jiang Q, Lu W, Li X. Tian Y, et al. Open Biol. 2021 Dec;11(12):210268. doi: 10.1098/rsob.210268. Epub 2021 Dec 1. Open Biol. 2021. PMID: 34847773 Free PMC article. Review.
References
- Sabbah HN: Apoptotic cell death in heart failure. Cardiovasc Res 2000, 45:704-712 - PubMed
- Fukuda K: Development of regenerative cardiomyocytes from mesenchymal stem cells for cardiovascular tissue engineering. Artif Organs 2001, 25:187-193 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases