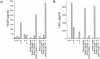An angiogenic switch in macrophages involving synergy between Toll-like receptors 2, 4, 7, and 9 and adenosine A(2A) receptors - PubMed (original) (raw)
An angiogenic switch in macrophages involving synergy between Toll-like receptors 2, 4, 7, and 9 and adenosine A(2A) receptors
Grace Pinhal-Enfield et al. Am J Pathol. 2003 Aug.
Abstract
Adenosine A(2A) receptor (A(2A)R) agonists synergize with Escherichia coli (E. coli) LPS [toll-like receptor (TLR)4 agonist] to up-regulate vascular endothelial growth factor (VEGF) expression in murine macrophages. Here, we demonstrate that TLR2, TLR7, and TLR9, but not TLR3 and TLR5 agonists, also synergize with A(2A)R agonists and adenosine to up-regulate VEGF, while simultaneously strongly down-regulating TNFalpha expression. In the absence of adenosine or A(2A)R agonists, Porphyromonas gingivalis (P. gingivalis) LPS and PAM(3)CAG (TLR2 agonists), resiquimod (R848) (TLR7 agonist), and non-methylated CpG DNA (TLR9 agonist) strongly up-regulate TNFalpha expression, with no effect on VEGF. In the presence of adenosine or A(2A)R agonists, but not A(1)R agonists, TLR2, 4, 7, and 9 agonists strongly up-regulate VEGF expression, while simultaneously down-regulating TNFalpha. C57BL/10ScN (TLR4 deletion mutant) macrophages produce TNFalpha in response to TLR2, 3, 7, and 9 agonists, but not the TLR4 agonist E. coli LPS. With adenosine or A(2A)R agonists, TLR2, 7, and 9, but not TLR4 agonists, also synergistically up-regulate VEGF, while down-regulating TNFalpha expression. Polyinosinic-polycytidilic acid (poly(I:C)) (TLR3 agonist) stimulates TNFalpha expression in macrophages from both C57BL/10ScSn and C57BL/10ScN mice, but has little effect on VEGF expression in the presence of adenosine or A(2A)R agonists. R-flagellins from Serratia marcescens (S. marcescens) and Salmonella muenchen (S. muenchen) do not stimulate TNFalpha expression in either C57BL/10ScSn or C57BL10/ScN mice, and have no effect on VEGF production in the presence of adenosine or A(2A)R agonists. While adenosine and A(2A)R agonists strongly down-regulate TNFalpha protein expression induced by TLR2, 3, 4, 7, and 9 agonists, TNFalpha mRNA and NF-kappaB activation are not reduced. We propose a novel signaling pathway in murine macrophages involving synergy between TLRs 2, 4, 7, and 9 and A(2A)Rs, that up-regulates VEGF and down-regulates TNFalpha expression, thus acting as an angiogenic switch. This angiogenic switch may play an important role in ischemia when TLR agonists are present, providing an interface between innate immunity and wound healing.
Figures
Figure 1.
Effects of taxol on TNFα (A) and VEGF (B) production by macrophages from C57BL/10SnSc and C57BL/10ScN mice. Macrophages from either C57BL/10ScSn (TLR4+/+) or C57BL/10ScN (TLR4−/−) mice were treated with taxol, E. coli LPS, or NECA at the indicated concentrations, either alone or in combination. Test agents were added to the macrophage cultures 18 hours after plating, and conditioned media were harvested 24 hours later, and assayed for TNFα and VEGF levels. At least two replicates were studied for each condition, and each sample was analyzed in duplicate. Results are presented as means ± SD. An asterisk (*) indicates undetectable levels.
Figure 2.
Effects of adenosine and an adenosine deaminase inhibitor (EHNA) on production of VEGF (A) and TNFα (B) by macrophages from C57Bl/10ScSn mice. Macrophages were treated with adenosine (A) (100 μmol/L), NECA (N) (1 μmol/L), LPS (L) (100 ng/ml), or EHNA at the indicated concentrations, either alone or in combination. Conditioned media were harvested 24 hours after addition of test agents and the TNFα and VEGF levels in the media were determined by ELISA. At least two replicates were studied for each condition, and each sample was analyzed for TNFα and VEGF in duplicate. Results are presented as means ± SD. An asterisk (*) indicates undetectable levels.
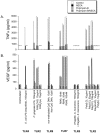
Figure 3.
Effects of TLR agonists on TNFα (A) and VEGF (B) production by macrophages from C57BL/10ScSn (TLR4+/+) mice. Macrophages were treated with TLR agonists and controls at the indicated concentrations. NECA (an A2R agonist) and polymyxin B were added to the cells at the same time as the TLR agonists. Conditioned media were harvested 24 hours after addition of test agents and the TNFα and VEGF levels in the media were determined by ELISA. At least two replicates were studied for each condition, and each sample was analyzed for TNFα and VEGF in duplicate. Results are presented as means ± SD. The x-axis labels refer to both TNFα (A) and VEGF (B). The particular TLR studied in each group is indicated beneath the x-axis labels. An asterisk (*) indicates undetectable levels.
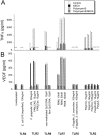
Figure 4.
Effects of TLR agonists on TNFα (A) and VEGF (B) protein production by macrophages from C57BL/10ScN (TLR4-deficient) mice. Macrophages were treated with TLR agonists and controls at the indicated concentrations. NECA (an A2R agonist) and polymyxin B were added to the cells at the same time as the TLR agonists. Conditioned media were harvested 24 hours after addition of test agents and the TNFα and VEGF levels in the media were determined by ELISA. At least two replicates were studied for each condition, and each sample was analyzed for TNFα and VEGF in duplicate. Results are presented as means ± SD. The x-axis labels refer to both TNFα (A) and VEGF (B). The particular TLR studied in each group is indicated beneath the x-axis labels. An asterisk (*) indicates undetectable levels.
Figure 5.
Northern blots showing the effect of E. coli LPS (TLR4 agonist) alone and with NECA (A2R agonist) on the steady-state levels of TNFα mRNA in macrophages from C57BL/10ScSn mice. Macrophages were treated for 2 and 4 hours, total RNA isolated, and 10 μg total RNA electrophoresed per lane through 1.2% agarose/formaldehyde gels. The RNA was then transferred to nylon membranes, and hybridized with an α32P-dCTP-labeled TNFα cDNA probe. Equal loading was confirmed by probing blots with a 32P-labeled 18S ribosomal RNA probe. C, untreated cells; L, E. coli LPS (100 ng/ml)-treated cells; N, NECA (1 μmol/L)-treated cells; L/N, LPS/NECA-treated cells.
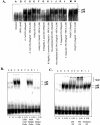
Figure 6.
EMSAs showing the effects of TLR agonists alone and with NECA (A2R agonist) on NF-κB activation in macrophages from C57BL/10ScSn mice. A: Macrophages were treated with TLR agonists and controls, with or without NECA for 1 hour (lanes A–N). Cells were harvested, nuclear protein extracts were prepared, and equal amounts of nuclear proteins were analyzed for NF-κB activation. B: Macrophages were treated with E. coli LPS (TLR4 agonist) for 1 hour, with or without NECA (1 μmol/L). Untreated cells are shown in lane A. Specificity of the activated NF-κB signal was determined by incubating in the presence of a 100-fold excess of unlabeled oligonucleotide as a competitor to inhibit the binding of NF-κB (lanes E and F) or 100X excess of a mutant probe (lanes G and H). Binding of the 32P-labeled mutant probe was also assessed (lanes I and J). C: Macrophages were treated with E. coli LPS with or without NECA (1 μmol/L) for 1 hour. Lane A: Untreated control. Lane B: NECA alone. Lane C: LPS alone. Lane D: LPS + NECA. Supershift analysis using specific polyclonal antibodies to the p50 and p65 subunits of NF-κB are shown in lanes E/F and G/H, respectively, while the effects of a non-specific antibody are shown in lanes I/J. C, untreated cells; L, LPS (100 ng/ml)-treated cells; N, NECA (1 μmol/L)-treated cells; L/N, LPS/NECA-treated cells.
Similar articles
- Synergistic up-regulation of vascular endothelial growth factor expression in murine macrophages by adenosine A(2A) receptor agonists and endotoxin.
Leibovich SJ, Chen JF, Pinhal-Enfield G, Belem PC, Elson G, Rosania A, Ramanathan M, Montesinos C, Jacobson M, Schwarzschild MA, Fink JS, Cronstein B. Leibovich SJ, et al. Am J Pathol. 2002 Jun;160(6):2231-44. doi: 10.1016/S0002-9440(10)61170-4. Am J Pathol. 2002. PMID: 12057925 Free PMC article. - Differential regulation of HIF-1alpha isoforms in murine macrophages by TLR4 and adenosine A(2A) receptor agonists.
Ramanathan M, Luo W, Csóka B, Haskó G, Lukashev D, Sitkovsky MV, Leibovich SJ. Ramanathan M, et al. J Leukoc Biol. 2009 Sep;86(3):681-9. doi: 10.1189/jlb.0109021. Epub 2009 May 28. J Leukoc Biol. 2009. PMID: 19477908 Free PMC article. - Induction of in vitro reprogramming by Toll-like receptor (TLR)2 and TLR4 agonists in murine macrophages: effects of TLR "homotolerance" versus "heterotolerance" on NF-kappa B signaling pathway components.
Dobrovolskaia MA, Medvedev AE, Thomas KE, Cuesta N, Toshchakov V, Ren T, Cody MJ, Michalek SM, Rice NR, Vogel SN. Dobrovolskaia MA, et al. J Immunol. 2003 Jan 1;170(1):508-19. doi: 10.4049/jimmunol.170.1.508. J Immunol. 2003. PMID: 12496438 - Adenosine receptors and mammalian toll-like receptors: synergism in macrophages.
Olah ME, Caldwell CC. Olah ME, et al. Mol Interv. 2003 Oct;3(7):370-4. doi: 10.1124/mi.3.7.370. Mol Interv. 2003. PMID: 14993458 Review. - Toll-like receptors as adjuvant receptors.
Kaisho T, Akira S. Kaisho T, et al. Biochim Biophys Acta. 2002 Feb 13;1589(1):1-13. doi: 10.1016/s0167-4889(01)00182-3. Biochim Biophys Acta. 2002. PMID: 11909637 Review.
Cited by
- Adenosine, bridging chronic inflammation and tumor growth.
Chen L, Alabdullah M, Mahnke K. Chen L, et al. Front Immunol. 2023 Oct 31;14:1258637. doi: 10.3389/fimmu.2023.1258637. eCollection 2023. Front Immunol. 2023. PMID: 38022572 Free PMC article. Review. - Macrophages and the maintenance of homeostasis.
Mosser DM, Hamidzadeh K, Goncalves R. Mosser DM, et al. Cell Mol Immunol. 2021 Mar;18(3):579-587. doi: 10.1038/s41423-020-00541-3. Epub 2020 Sep 15. Cell Mol Immunol. 2021. PMID: 32934339 Free PMC article. Review. - Suppression of PLCbeta2 by endotoxin plays a role in the adenosine A(2A) receptor-mediated switch of macrophages from an inflammatory to an angiogenic phenotype.
Grinberg S, Hasko G, Wu D, Leibovich SJ. Grinberg S, et al. Am J Pathol. 2009 Dec;175(6):2439-53. doi: 10.2353/ajpath.2009.090290. Epub 2009 Oct 22. Am J Pathol. 2009. PMID: 19850892 Free PMC article. - Invited Lectures : Overviews Purinergic signalling: past, present and future.
[No authors listed] [No authors listed] Purinergic Signal. 2006 May;2(1):1-324. doi: 10.1007/s11302-006-9006-2. Epub 2006 May 15. Purinergic Signal. 2006. PMID: 18404494 Free PMC article. No abstract available. - Targeting of G-protein coupled receptors in sepsis.
Rehman A, Baloch NU, Morrow JP, Pacher P, Haskó G. Rehman A, et al. Pharmacol Ther. 2020 Jul;211:107529. doi: 10.1016/j.pharmthera.2020.107529. Epub 2020 Mar 19. Pharmacol Ther. 2020. PMID: 32197794 Free PMC article. Review.
References
- Ferrara N, Davis-Smyth T: The biology of vascular endothelial growth factor. Endocr Rev 1997, 18:4-25 - PubMed
- Dvorak HF, Nagy JA, Feng D, Brown LF, Dvorak AM: Vascular permeability factor/vascular endothelial growth factor and the significance of microvascular hyperpermeability in angiogenesis. Curr Top Microbiol Immunol 1999, 237:97-132 - PubMed
- Leibovich SJ, Chen JF, Pinhal-Enfield G, Belem PC, Elson G, Rosania A, Ramanathan M, Montesinos C, Jacobson M, Schwarzschild MA, Fink JS, Cronstein B: Synergistic up-regulation of vascular endothelial growth factor expression in murine macrophages by adenosine A2A receptor agonists and endotoxin. Am J Pathol 2002, 160:2231-2244 - PMC - PubMed
- Means TK, Golenbock DT, Fenton MJ: Structure and function of toll-like receptor proteins. Life Sci 2000, 68:241-258 - PubMed
- Underhill DM, Ozinsky A: Toll-like receptors: key mediators of microbe detection. Curr Opin Immunol 2002, 14:103-110 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous