Phosphorylation-independent desensitization of GABA(B) receptor by GRK4 - PubMed (original) (raw)
Phosphorylation-independent desensitization of GABA(B) receptor by GRK4
Julie Perroy et al. EMBO J. 2003.
Abstract
Agonist-promoted desensitization of the heterodimeric metabotropic GABA(B) receptor was investigated. Whereas no desensitization was observed in HEK293 cells heterologously expressing the receptor, GABA and the synthetic agonist baclofen induced a robust desensitization in cerebellar granule cells endogenously expressing the receptor. Taking advantage of this cell-specific desensitization phenotype, we identified GRK4 as the kinase involved in the neuronal desensitization. Transfection of small interference RNA directed against GRK4 significantly reduced GRK4 levels in cerebellar granule cells and strongly inhibited the agonist-promoted desensitization. Reciprocally, transfection of GRK4 in HEK293 cells restored agonist-promoted desensitization, confirming that this kinase is sufficient to support desensitization. Surprisingly, this desensitization occurred in the absence of ligand-induced receptor phosphorylation and could be promoted by GRK4 mutants deleted of their kinase domain. Taken together, these results suggest that GRK4 plays a central role in the agonist-promoted desensitization of GABA(B) receptor and that it does so through an atypical mechanism that challenges the generally accepted model linking the kinase activity of GRKs to their role in receptor desensitization.
Figures
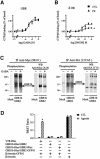
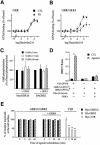
Fig. 1. Lack of agonist induced desensitization of GBR in HEK293 cells. (A and B) Agonist-stimulated [35S]GTPγS binding was measured in membranes derived from HEK293 cells expressing Myc-GBR1b/HA-GBR2 (A) or Myc-δOR (B) pre-stimulated (PS; triangles) or not (CTL; squares) for 60 min with the specific agonist [1 mM GABA (A) or 10 µM SNC80 (B)]. Data were analyzed by non-linear regression (GraphPad Prism) and are presented as the mean ± SEM of at least three independent experiments performed in triplicate. *P < 0.05 between the asymptotes (maximal response) of the curves. (C) The phosphorylation state of Myc-GBR1b and HA-GBR2 was studied following metabolic labeling with [32P]Pi. Cells transfected with pcDNA3 alone (mock) or Myc-GBR1b/HA-GBR2 were incubated (+) or not (–) with 1 mM GABA for 60 min and the receptor purified by immunoprecipitation (IP) using mouse anti-Myc (9E10) or anti-HA (12CA5) antibodies. Immunocomplexes were analyzed by SDS–PAGE and autoradiography. The identity of the bands was confirmed by western blot analysis (WB) on the same gels using rabbit anti-Myc (A14) or anti-HA (Y11) antibodies. The autoradiograms shown are representative of three independent experiments. Arrows indicate the phosphorylated forms of Myc-GBR1b and HA-GBR2. (D) Recruitment of β-arrestin in living cells. HEK293 cells were transiently co-transfected with Myc-V2R-_R_luc or Myc-GBR1b-_R_luc/HA-GBR2-_R_luc in combination with β-arrestin-GFP10. For each pair considered, the quantities of DNA used for Myc-V2R-_R_luc or Myc-GBR1b-_R_luc/HA-GBR2-_R_luc were selected to yield equivalent luminescent signals (370–450 nm), whereas that for β-arrestin-GFP10 was selected to obtain the maximum BRET levels. Cells were treated (agonist) or not (CTL) with the appropriate selective agonists (1 µM Arg-vasopressin and 1 mM GABA). Following 30 min incubation, the energy transfer reaction was initiated by adding DeepBlueC to each well and BRET assessed in a BRETCount microplate reader. The results represent the mean ± SEM of three to six independent experiments performed in triplicate. *P < 0.05.
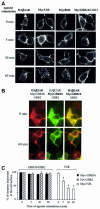
Fig. 2. Lack of agonist-induced internalization of GBR in HEK293 cells. (A and B) Internalization of HA-β2AR, Myc-V2R, Myc-δOR or Myc-GBR1b/GBR2 expressed individually (A) and of Myc-GBR1b/GBR2 and HA-β2AR expressed simultaneously (B) was assessed by immunofluorescence microscopy. Intact cells were incubated with anti-Myc (9E10) or anti-HA (Y11) antibodies before stimulation with their specific agonists for the indicated time (β2AR: 1 µM isoproterenol; V2R: 1 µM Arg-vasopressin; δOR: 10 µM SNC80; and GBR: 1 mM GABA). Immunoreactivity was revealed with the appropriate Alexa488- or Alexa594-conjugated secondary antibodies. Characters in bold indicate the visualized receptors. Pictures shown are representative of five independent experiments. (C) ELISA was performed to quantify cell surface receptor expression following agonist stimulation. Results are expressed as a percentage of N-terminally tagged Myc-GBR1b/HA-GBR2 and Myc-V2R remaining at the cell surface following the indicated time of agonist stimulation, 0 min being taken as 100%. Data represent the mean ± SEM of three independent experiments. *P < 0.05.
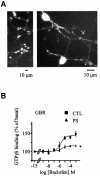
Fig. 3. Desensitization of GBR in cerebellar granule cell. (A) Endogenously expressed GBRs were detected in cerebellar granule cells (GCG) by immunofluorescence microscopy. Permeabilized cells were immunolabelled using a polyclonal antibody raised against native GBR2. (B) Baclofen-stimulated [35S]GTPγS binding was measured in membranes derived from CGC, pre-stimulated (PS; triangles) or not (CTL; squares) for 60 min with the selective agonist (1 mM baclofen). Data represent the mean ± SEM of three independent experiments performed in triplicate. *P < 0.05.
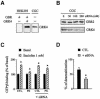
Fig. 4. The GRK4 protein is required for GBR desensitization in cerebellar granule cells. (A) Total GRK4 immunoreactivity was assessed in total lysate from HEK293 cells (transfected with Myc-GBR1b/HA-GBR2 alone or with GRK4) and in native cerebellar granule cells (CGC). (B) Total GBR2 and GRK4 immunoreactivity detected in total lysate from CGC transfected or not with the indicated amount of GRK4-siRNA. In (A) and (B), proteins were detected following SDS–PAGE and immunoblotting using rabbit anti-GRK4 (H70) or rabbit anti-GBR2 antibodies. The western blots shown are representative of three independent experiments. (C) Baclofen-stimulated [35S]GTPγS binding was measured in membranes of CGC transfected or not with 100 nM GRK4-siRNA and pre-stimulated (PS) or not (CTL) for 60 min with 1 mM baclofen. Data represent the mean ± SEM of three independent experiments performed in triplicate. (D) The desensitization of the responses measured in (C) was expressed as the decrease in baclofen-stimulated response following pre-stimulation. The data are expressed as percentage of the control response. *P < 0.05.
Fig. 5. GRK4 promotes phosphorylation-independent desensitization of GBR in HEK293. (A and B) Baclofen-stimulated [35S]GTPγS binding was measured in membranes of HEK293 cells transfected with Myc-GBR1b/HA-GBR2 (A) or in combination with GRK4 (B) pre- stimulated (PS; triangles) or not (CTL; squares) for 60 min with the specific agonist (1 mM baclofen). Data represent the mean ± SEM of three independent experiments performed in triplicate. *P < 0.05. (C) Receptor phosphorylation was assessed following [32P]Pi metabolic labeling of HEK293 cells co-transfected with Myc-GBR1b/HA-GBR2 in the presence or absence of GRK4. Cells were either stimulated or not with 1 mM GABA for the indicated times. GBR1 and GBR2 were then purified by immunoprecipitation and resolved on SDS–PAGE as in Figure 1C. GBR phosphorylation is expressed as a percentage of the phosphorylation level observed in the absence of agonist and was normalized as a function of the total amount of purified receptor detected by western blot analysis carried out on the same blots. Data shown are the mean ± SEM of three independent experiments. (D) Recruitment of β-arrestin in living cells. HEK293 cells were transiently transfected with the indicated combination of plasmids. BRET experiments were then carried out as in Figure 1D. The results represent the mean ± SEM of three to six independent experiments performed in triplicate. (E) ELISA was performed to quantify cell surface receptor expression following agonist stimulation as in Figure 2C. *P < 0.05.
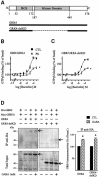
Fig. 6. GRK4 interacts with and promotes the desensitization of GBR independently of its kinase domain. (A) Topological organization of GRK4 and GRK4-delKD. (B and C) Baclofen-stimulated [35S]GTPγS binding was measured in membranes of HEK293 cells transfected with Myc-GBR1b/HA-GBR2 and GRK4 (B) or GRK4-delKD (C) pre- stimulated (PS; triangles) or not (CTL; squares) for 60 min with the specific agonist (1 mM baclofen). Data represent the mean ± SEM of three experiments performed in triplicate. *P < 0.05. (D) Left panel, co-immunoprecipitation of GRK4 and GRK4-delKD with HA-GBR2 was carried out in HEK293 cell lysates transfected with the indicated plasmids. Immunoprecipitation (IP) using anti-HA (12CA5) antibody was performed on total cell extracts pre-treated or not with 1 mM GABA for 60 min. The immunocomplexes were then analyzed by western blot analysis using antibody (H70) raised against the N-terminal part of GRK4 (common to both GRK4 constructs). Right panel: following HA-GBR2 IP, GRK4 immunoreactivity was quantified by laser densitometry and the data analyzed using Quantity One (Bio-Rad). Arrows indicate the bands corresponding to GRK4 and GRK4-delKD. Data shown are the mean ± SEM of three independent experiments. *P < 0.05.
Similar articles
- Desensitization of GABA(B) receptor signaling by formation of protein complexes of GABA(B2) subunit with GRK4 or GRK5.
Kanaide M, Uezono Y, Matsumoto M, Hojo M, Ando Y, Sudo Y, Sumikawa K, Taniyama K. Kanaide M, et al. J Cell Physiol. 2007 Jan;210(1):237-45. doi: 10.1002/jcp.20863. J Cell Physiol. 2007. PMID: 17013811 - Novel features of G protein-coupled receptor kinase 4.
Neve KA. Neve KA. Mol Pharmacol. 2006 Mar;69(3):673-6. doi: 10.1124/mol.105.021535. Epub 2005 Dec 9. Mol Pharmacol. 2006. PMID: 16339846 - Phosphorylation and desensitization of the human thromboxane receptor-alpha by G protein-coupled receptor kinases.
Zhou H, Yan F, Tai HH. Zhou H, et al. J Pharmacol Exp Ther. 2001 Sep;298(3):1243-51. J Pharmacol Exp Ther. 2001. PMID: 11504827 - [Mechanisms of regulation and function of G-protein coupled receptor kinases].
Sobierajska K, Fabczak H, Fabczak S. Sobierajska K, et al. Postepy Biochem. 2005;51(4):421-9. Postepy Biochem. 2005. PMID: 16676577 Review. Polish. - Functional modulation of GABAB receptors by protein kinases and receptor trafficking.
Terunuma M, Pangalos MN, Moss SJ. Terunuma M, et al. Adv Pharmacol. 2010;58:113-22. doi: 10.1016/S1054-3589(10)58005-0. Adv Pharmacol. 2010. PMID: 20655480 Free PMC article. Review.
Cited by
- Reversal of inhibition of putative dopaminergic neurons of the ventral tegmental area: interaction of GABA(B) and D2 receptors.
Nimitvilai S, Arora DS, McElvain MA, Brodie MS. Nimitvilai S, et al. Neuroscience. 2012 Dec 13;226:29-39. doi: 10.1016/j.neuroscience.2012.08.045. Epub 2012 Sep 15. Neuroscience. 2012. PMID: 22986166 Free PMC article. - Monitoring agonist-promoted conformational changes of beta-arrestin in living cells by intramolecular BRET.
Charest PG, Terrillon S, Bouvier M. Charest PG, et al. EMBO Rep. 2005 Apr;6(4):334-40. doi: 10.1038/sj.embor.7400373. EMBO Rep. 2005. PMID: 15776020 Free PMC article. - Effects of decreased renal cortical expression of G protein-coupled receptor kinase 4 and angiotensin type 1 receptors in rats.
Yatabe J, Sanada H, Midorikawa S, Hashimoto S, Watanabe T, Andrews PM, Armando I, Wang X, Felder RA, Jose PA. Yatabe J, et al. Hypertens Res. 2008 Jul;31(7):1455-64. doi: 10.1291/hypres.31.1455. Hypertens Res. 2008. PMID: 18957817 Free PMC article. - Paroxetine alleviates rat limb post-ischemia induced allodynia through GRK2 upregulation in superior cervical ganglia.
Tang J, Dong J, Yang L, Gao L, Zheng J. Tang J, et al. Int J Clin Exp Med. 2015 Feb 15;8(2):2065-76. eCollection 2015. Int J Clin Exp Med. 2015. PMID: 25932137 Free PMC article. - Opposite effects of KCTD subunit domains on GABA(B) receptor-mediated desensitization.
Seddik R, Jungblut SP, Silander OK, Rajalu M, Fritzius T, Besseyrias V, Jacquier V, Fakler B, Gassmann M, Bettler B. Seddik R, et al. J Biol Chem. 2012 Nov 16;287(47):39869-77. doi: 10.1074/jbc.M112.412767. Epub 2012 Oct 3. J Biol Chem. 2012. PMID: 23035119 Free PMC article.
References
- Ambrose C., James,M., Barnes,G., Lin,C., Bates,G., Altherr,M., Duyao,M., Groot,N., Church,D. and Wasmuth,J.J. (1992) A novel G protein-coupled receptor kinase gene cloned from 4p16.3. Hum. Mol. Genet., 1, 697–703. - PubMed
- Ango F., Albani-Torregrossa,S., Joly,C., Robbe,D., Michel,J.M., Pin,J.P., Bockaert,J. and Fagni,L. (1999) A simple method to transfer plasmid DNA into neuronal primary cultures: functional expression of the mGlu5 receptor in cerebellar granule cells. Neuropharmacology, 38, 793–803. - PubMed
- Billinton A., Ige,A.O., Bolam,J.P., White,J.H., Marshall,F.H. and Emson,P.C. (2001) Advances in the molecular understanding of GABAB receptors. Trends Neurosci., 24, 277–282. - PubMed
- Carman C.V., Lisanti,M.P. and Benovic,J.L. (1999a) Regulation of G protein-coupled receptor kinases by caveolin. J. Biol. Chem., 274, 8858–8864. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources