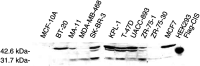Suppressor of cytokine signalling gene expression is elevated in breast carcinoma - PubMed (original) (raw)
Suppressor of cytokine signalling gene expression is elevated in breast carcinoma
M Raccurt et al. Br J Cancer. 2003.
Abstract
Cytokines are important for breast cell function, both as trophic hormones and as mediators of host defense mechanisms against breast cancer. Recently, inducible feedback suppressors of cytokine signalling (SOCS/JAB/SSI) have been identified, which decrease cell sensitivity to cytokines. We examined the expression of SOCS genes in 17 breast carcinomas and 10 breast cancer lines, in comparison with normal tissue and breast lines. We report elevated expression of SOCS-1-3 and CIS immunoreactive proteins within in situ ductal carcinomas and infiltrating ductal carcinomas relative to normal breast tissue. Significantly increased expression of SOCS-1-3 and CIS transcripts was also shown by quantitative in situ hybridisation within both tumour tissue and reactive stroma. CIS transcript expression was elevated in all 10 cancer lines, but not in control lines. However, there was no consistent elevation of other SOCS transcripts. CIS protein was shown by immunoblot to be present in all cancer lines at increased levels, mainly as the 47 kDa ubiquitinylated form. A potential proliferative role for CIS overexpression is supported by reports that CIS activates ERK kinases, and by strong induction in transient reporter assays with an ERK-responsive promoter. The in vivo elevation of SOCS gene expression may be part of the host/tumour response or a response to autocrine/paracrine GH and prolactin. However, increased CIS expression in breast cancer lines appears to be a specific lesion, and could simultaneously shut down STAT 5 signalling by trophic hormones, confer resistance to host cytokines and increase proliferation through ERK kinases.
Figures
Figure 1
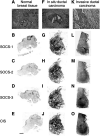
Macroautoradiographic pattern of ISH of SOCS-1–3, CIS mRNA performed on sections of normal breast (A–E) and typical lesions of in situ (F–J) and invasive (K–O) ductal carcinomas. Semiquantitative expression of mRNA was performed on typical areas of the samples, as illustrated in B, G and L, after precise microscopic examination of the contiguous HPS-stained sections (A, F and K). The signal (dark areas) corresponds to different levels of SOCS and CIS genes expression. On adjacent sections of normal breast tissue, the signal obtained for the four genes is localised to the ducts and lobules (arrow) (B–E). On adjacent sections of in situ ductal carcinoma, the same intense signal is observed for the four genes and is localized to areas corresponding to enlarged ducts and periductal stroma reaction (arrows) (G–J). On adjacent sections of invasive ductal carcinoma, the increased density observed with the four probes encompasses the area corresponding to tumour cells infiltrating the cellular stroma (L–O). Thus, a more intense signal is seen specifically localised to the area of tumour invasion (arrow). Bar, 5 mm.
Figure 2
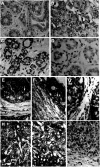
Cellular expression of SOCS-1–3 and CIS mRNA in normal breast (A, C, D), in situ (E–G) and invasive (H–J) ductal breast carcinomas, evidenced by the presence of bright silver grains on emulsion-coated sections. On sections of normal breast tissue, basal levels of SOCS-1 (C) and CIS (D) were detected in normal epithelial cells and in scattered fibroblasts of the surrounding connective tissue. On sections from patients with in situ ductal carcinoma, expression of SOCS-1 (E), SOCS-2 (F) and CIS (G) transcripts was strongly associated with proliferative tumour cells (arrows) of the enlarged ducts, in concentric layers of fibroblastic cells (arrowheads) and in lymphocytes of inflammatory infiltrates (*). On sections from patients with invasive ductal carcinoma, gene expression of SOCS-1 (H), SOCS-3 (I) and CIS (J) was abundantly detected in the whole area of the tumour. The close association of cancerous cells and stromal cells prevents the precise identification of the positive cell component. No signal was observed when in situ hybridisation was performed with heterologous cDNA probe as a negative control on normal breast tissue (A) and invasive ductal carcinoma (B). Bar, 50 _μ_m.
Figure 3
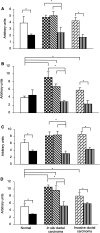
Quantification of mRNA levels was performed on macroautoradiograms from normal breast, in situ and invasive ductal carcinomas as described in Materials and Methods. Optical densities were measured for the four probes (A: SOCS-1, B: SOCS-2, C: SOCS-3, D: CIS) in typical areas according to pathological criteria: (□), normal duct; (▪), normal connective tissue; ( ), cancerous ducts; (
), cancerous ducts; ( ), reactive stroma; (
), reactive stroma; ( ), invasive area; (
), invasive area; ( ), adjacent normal connective tissue; expressed in arbitrary units±s.e.m. *P<0.05.
), adjacent normal connective tissue; expressed in arbitrary units±s.e.m. *P<0.05.
Figure 4
Immunostaining for CIS and SOCS-3 in normal breast tissue and infiltrating ductal carcinoma. Tissues were fixed and processed as described in the Materials and Methods section. Cancerous cells were strongly immunoreactive for CIS (A1) and SOCS-3 (B1) when compared with normal tissues (A2 and B2, respectively). Arrows indicate equal immunoreactivity in blood vessels of normal and cancerous tissue. Bar, 100 _μ_m.
Figure 5
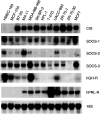
Expression of transcripts for CIS and SOCS-1–3 and for GH and prolactin receptors in normal (HMEC184 and MCF-10A) and 10 breast cancer lines. Total RNA was extracted from cell lines, subjected to electrophoresis on a formaldehyde gel and blotted on a nylon membrane. Identical blots were run and each was hybridized with a specific cDNA probe. The blots were then washed and exposed to X-ray films as described in Materials and Methods. The exposure time for CIS was 2 days; for SOCS-1, 5 days; for SOCS-2, 5 days; and for SOCS-3, 3 days. Blots were stripped and reprobed for 18S rRNA to ensure equal loading.
Figure 6
CIS protein expression in breast cancer lines. Total cell lysate was obtained from confluent cultures of breast cancer lines or normal lines. Lysates were then immunobloted and probed with goat anti-CIS antibody as described in the Materials and Methods. FLAG-tagged CIS expressed in HEK 293 cells was used as a positive control. Signal corresponding to the CIS protein was detected migrating at 32 kDa in breast cancer cell lines. Another slower migrating band at 47 kDa corresponds to the ubiquitinylated form of CIS.
Figure 7
Lack of effect of serum on expression of CIS protein in breast cancer lines. Cells were exposed to 10% serum or serum starved for 12 h before harvesting for immunoblot analyses as for Figure 6.
Figure 8
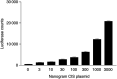
CIS transfection results in elevated ERK reporter activity. CHO cells were transfected with increasing amounts of CIS expression plasmid and an ERK reporter plasmid. The total amount of DNA was normalised to 3 _μ_g with empty pcDNA3. Luciferase assay was carried out as described in the Materials and Methods section. Luciferase counts expressed as mean±s.e.m., four replicates per point. This result was obtained on three separate occasions.
Similar articles
- Suppressors of cytokine signalling: SOCS.
Larsen L, Röpke C. Larsen L, et al. APMIS. 2002 Dec;110(12):833-44. doi: 10.1034/j.1600-0463.2002.1101201.x. APMIS. 2002. PMID: 12645661 Review. - Inhibition and restoration of prolactin signal transduction by suppressors of cytokine signaling.
Pezet A, Favre H, Kelly PA, Edery M. Pezet A, et al. J Biol Chem. 1999 Aug 27;274(35):24497-502. doi: 10.1074/jbc.274.35.24497. J Biol Chem. 1999. PMID: 10455112 - Induction of JAB/SOCS-1/SSI-1 and CIS3/SOCS-3/SSI-3 is involved in gp130 resistance in cardiovascular system in rat treated with cardiotrophin-1 in vivo.
Hamanaka I, Saito Y, Yasukawa H, Kishimoto I, Kuwahara K, Miyamoto Y, Harada M, Ogawa E, Kajiyama N, Takahashi N, Izumi T, Kawakami R, Masuda I, Yoshimura A, Nakao K. Hamanaka I, et al. Circ Res. 2001 Apr 13;88(7):727-32. doi: 10.1161/hh0701.088512. Circ Res. 2001. PMID: 11304496 - SOCS: suppressors of cytokine signalling.
Starr R, Hilton DJ. Starr R, et al. Int J Biochem Cell Biol. 1998 Oct;30(10):1081-5. doi: 10.1016/s1357-2725(98)00067-3. Int J Biochem Cell Biol. 1998. PMID: 9785473 Review.
Cited by
- Suppressor of cytokine signalling 2 (SOCS-2) expression in breast carcinoma.
Farabegoli F, Ceccarelli C, Santini D, Taffurelli M. Farabegoli F, et al. J Clin Pathol. 2005 Oct;58(10):1046-50. doi: 10.1136/jcp.2004.024919. J Clin Pathol. 2005. PMID: 16189149 Free PMC article. - Signal transducer and activator of transcription 5A/B in prostate and breast cancers.
Tan SH, Nevalainen MT. Tan SH, et al. Endocr Relat Cancer. 2008 Jun;15(2):367-90. doi: 10.1677/ERC-08-0013. Endocr Relat Cancer. 2008. PMID: 18508994 Free PMC article. Review. - Suppressor of cytokine signaling-3 antagonizes cAMP effects on proliferation and apoptosis and is expressed in human prostate cancer.
Bellezza I, Neuwirt H, Nemes C, Cavarretta IT, Puhr M, Steiner H, Minelli A, Bartsch G, Offner F, Hobisch A, Doppler W, Culig Z. Bellezza I, et al. Am J Pathol. 2006 Dec;169(6):2199-208. doi: 10.2353/ajpath.2006.060171. Am J Pathol. 2006. PMID: 17148681 Free PMC article. - HMGN2 inducibly binds a novel transactivation domain in nuclear PRLr to coordinate Stat5a-mediated transcription.
Fiorillo AA, Medler TR, Feeney YB, Liu Y, Tommerdahl KL, Clevenger CV. Fiorillo AA, et al. Mol Endocrinol. 2011 Sep;25(9):1550-64. doi: 10.1210/me.2011-0106. Epub 2011 Aug 4. Mol Endocrinol. 2011. PMID: 21816901 Free PMC article. - Discrimination of Human Cell Lines by Infrared Spectroscopy and Mathematical Modeling.
Zendehdel R, H Shirazi F. Zendehdel R, et al. Iran J Pharm Res. 2015 Summer;14(3):803-10. Iran J Pharm Res. 2015. PMID: 26330868 Free PMC article.
References
- Adams TE, Hansen JA, Starr R, Nicola NA, Hilton DJ, Billestrup NJ (1998) Growth hormone preferentially induces the rapid, transient expression of SOCS-3, a novel inhibitor of cytokine receptor signaling. J Biol Chem 273: 1285–1287 - PubMed
- Allione A, Consalvo M, Nani P, Lollini PL, Cavallo F, Giovarelli M, Forni M, Gulino A, Colombo MP, Dellabona P (1994) Immunizing and curative potential of replicating and non-replicating murine mammary adenocarcinoma cells engineered with interleukins -2, -4, -6, -7, -10, tumor necrosis factor α, GMCSF and γ interferon gene or admixed with conventional adjuvants. Cancer Res 54: 6022–6026 - PubMed
- Argetsinger LS, Carter-Su C (1996) Mechanism of signaling by GH receptor. Physiol Rev 4: 1089–1107 - PubMed
- Boutin JM, Edery M, Shirota M, Jolicoeur C, Lesueur L, Ali S, Gould D, Djiane J, Kelly PA (1989) Identification of a cDNA encoding a long form of prolactin receptor in human hepatoma and breast cancer cells. Mol Endocrinol 3: 1455–1461 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous