A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer - PubMed (original) (raw)
Clinical Trial
A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer
James C Yang et al. N Engl J Med. 2003.
Abstract
Background: Mutations in the tumor-suppressor gene VHL cause oversecretion of vascular endothelial growth factor by clear-cell renal carcinomas. We conducted a clinical trial to evaluate bevacizumab, a neutralizing antibody against vascular endothelial growth factor, in patients with metastatic renal-cell carcinoma.
Methods: A randomized, double-blind, phase 2 trial was conducted comparing placebo with bevacizumab at doses of 3 and 10 mg per kilogram of body weight, given every two weeks; the time to progression of disease and the response rate were primary end points. Crossover from placebo to antibody treatment was allowed, and survival was a secondary end point.
Results: Minimal toxic effects were seen, with hypertension and asymptomatic proteinuria predominating. The trial was stopped after the interim analysis met the criteria for early stopping. With 116 patients randomly assigned to treatment groups (40 to placebo, 37 to low-dose antibody, and 39 to high-dose antibody), there was a significant prolongation of the time to progression of disease in the high-dose--antibody group as compared with the placebo group (hazard ratio, 2.55; P<0.001). There was a small difference, of borderline significance, between the time to progression of disease in the low-dose--antibody group and that in the placebo group (hazard ratio, 1.26; P=0.053). The probability of being progression-free for patients given high-dose antibody, low-dose--antibody, and placebo was 64 percent, 39 percent, and 20 percent, respectively, at four months and 30 percent, 14 percent, and 5 percent at eight months. At the last analysis, there were no significant differences in overall survival between groups (P>0.20 for all comparisons).
Conclusions: Bevacizumab can significantly prolong the time to progression of disease in patients with metastatic renal-cell cancer.
Copyright 2003 Massachusetts Medical Society
Figures
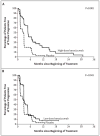
Figure 1. Kaplan–Meier Analysis of Survival Free of Tumor Progression for Patients Receiving High-Dose Bevacizumab (Panel A) or Low-Dose Bevacizumab (Panel B), as Compared with Placebo
The high dose of bevacizumab was 10 mg per kilogram of body weight. The low dose of bevacizumab was 3 mg per kilogram. Doses were given every two weeks. P values were calculated by the log-rank test.
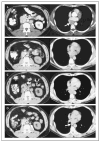
Figure 2. Serial Radiographs of a Patient Treated with High-Dose Bevacizumab
Panel A shows the pretreatment assessment (arrows indicate lymph-node metastases). Panel B shows a radiograph obtained two years later, when treatment was stopped during a partial response. Panel C shows relapse of tumor six months thereafter. Panel D shows a second partial response 3 months after therapy was restarted, which is ongoing at more than 18 months as of this writing.
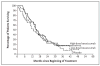
Figure 3. Overall Survival of Patients Receiving Placebo, Low-Dose Bevaci-zumab, or High-Dose Bevacizumab
There were no significant differences among the treatment groups.
Comment in
- The von Hippel-Lindau protein, vascular endothelial growth factor, and kidney cancer.
George DJ, Kaelin WG Jr. George DJ, et al. N Engl J Med. 2003 Jul 31;349(5):419-21. doi: 10.1056/NEJMp030061. N Engl J Med. 2003. PMID: 12890838 No abstract available. - Bevacizumab in renal-cell cancer.
Sonpavde G. Sonpavde G. N Engl J Med. 2003 Oct 23;349(17):1674. doi: 10.1056/NEJM200310233491719. N Engl J Med. 2003. PMID: 14573745 No abstract available. - New antiangiogenic agents for renal cell carcinoma: bevacizumab.
Bukowski RM. Bukowski RM. Curr Oncol Rep. 2004 Mar;6(2):85-6. doi: 10.1007/s11912-004-0017-2. Curr Oncol Rep. 2004. PMID: 14751083 No abstract available.
Similar articles
- The von Hippel-Lindau protein, vascular endothelial growth factor, and kidney cancer.
George DJ, Kaelin WG Jr. George DJ, et al. N Engl J Med. 2003 Jul 31;349(5):419-21. doi: 10.1056/NEJMp030061. N Engl J Med. 2003. PMID: 12890838 No abstract available. - Bevacizumab for patients with metastatic renal cancer: an update.
Yang JC. Yang JC. Clin Cancer Res. 2004 Sep 15;10(18 Pt 2):6367S-70S. doi: 10.1158/1078-0432.CCR-050006. Clin Cancer Res. 2004. PMID: 15448032 Review. - Cancer and Leukemia Group B 90206: A randomized phase III trial of interferon-alpha or interferon-alpha plus anti-vascular endothelial growth factor antibody (bevacizumab) in metastatic renal cell carcinoma.
Rini BI, Halabi S, Taylor J, Small EJ, Schilsky RL; Cancer and Leukemia Group B. Rini BI, et al. Clin Cancer Res. 2004 Apr 15;10(8):2584-6. doi: 10.1158/1078-0432.ccr-03-0605. Clin Cancer Res. 2004. PMID: 15102658 Clinical Trial. - Biology and clinical development of vascular endothelial growth factor-targeted therapy in renal cell carcinoma.
Rini BI, Small EJ. Rini BI, et al. J Clin Oncol. 2005 Feb 10;23(5):1028-43. doi: 10.1200/JCO.2005.01.186. Epub 2004 Nov 8. J Clin Oncol. 2005. PMID: 15534359 Review.
Cited by
- CT perfusion as an imaging biomarker in monitoring response to neoadjuvant bevacizumab and radiation in soft-tissue sarcomas: comparison with tumor morphology, circulating and tumor biomarkers, and gene expression.
Kambadakone A, Yoon SS, Kim TM, Karl DL, Duda DG, DeLaney TF, Sahani DV. Kambadakone A, et al. AJR Am J Roentgenol. 2015 Jan;204(1):W11-8. doi: 10.2214/AJR.13.12412. AJR Am J Roentgenol. 2015. PMID: 25539263 Free PMC article. Clinical Trial. - Differentiating mTOR inhibitors in renal cell carcinoma.
Pal SK, Quinn DI. Pal SK, et al. Cancer Treat Rev. 2013 Nov;39(7):709-19. doi: 10.1016/j.ctrv.2012.12.015. Epub 2013 Feb 21. Cancer Treat Rev. 2013. PMID: 23433636 Free PMC article. Review. - Nephrotoxicity of recent anti-cancer agents.
Lameire N. Lameire N. Clin Kidney J. 2014 Feb;7(1):11-22. doi: 10.1093/ckj/sft135. Epub 2013 Nov 26. Clin Kidney J. 2014. PMID: 25859345 Free PMC article. Review. - The effectiveness of RECIST on survival in patients with NSCLC receiving chemotherapy with or without target agents as first-line treatment.
Zhou T, Zheng L, Hu Z, Zhang Y, Fang W, Zhao Y, Ge J, Zhao H, Zhang L. Zhou T, et al. Sci Rep. 2015 Jan 8;5:7683. doi: 10.1038/srep07683. Sci Rep. 2015. PMID: 25567662 Free PMC article. - Cardiotoxicity of molecularly targeted agents.
Hedhli N, Russell KS. Hedhli N, et al. Curr Cardiol Rev. 2011 Nov;7(4):221-33. doi: 10.2174/157340311799960636. Curr Cardiol Rev. 2011. PMID: 22758623 Free PMC article. Review.
References
- Gnarra JR, Duan DR, Weng Y, et al. Molecular cloning of the von Hippel-Lindau tumor suppressor gene and its role in renal carcinoma. Biochim Biophys Acta. 1996;1242:201–10. - PubMed
- Gnarra JR, Tory K, Weng Y, et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet. 1994;7:85–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous