Autoinducer 2 production by Streptococcus gordonii DL1 and the biofilm phenotype of a luxS mutant are influenced by nutritional conditions - PubMed (original) (raw)
Autoinducer 2 production by Streptococcus gordonii DL1 and the biofilm phenotype of a luxS mutant are influenced by nutritional conditions
David S Blehert et al. J Bacteriol. 2003 Aug.
Abstract
The luxS gene, present in many bacterial genera, encodes the autoinducer 2 (AI-2) synthase. AI-2 has been implicated in bacterial signaling, and this study investigated its role in biofilm formation by Streptococcus gordonii, an organism that colonizes human tooth enamel within the first few hours after professional cleaning. Northern blotting and primer extension analyses revealed that S. gordonii luxS is monocistronic. AI-2 production was dependent on nutritional conditions, and maximum AI-2 induction was detected when S. gordonii was grown in the presence of serum and carbonate. In planktonic cultures, AI-2 production rose sharply during the transition from exponential to stationary phase, and the AI-2 concentration peaked approximately 4 h into stationary phase. An S. gordonii luxS mutant that did not produce AI-2 was constructed by homologous recombination. Complementation of the mutant by insertion of an intact luxS gene into the chromosome in tandem with the disrupted gene restored AI-2 production to a level similar to that of the wild-type strain. In planktonic culture, no growth differences were observed between the mutant and wild-type strains when five different media were used. However, when grown for 4 h as biofilms in 25% human saliva under flow, the luxS mutant formed tall microcolonies that differed from those formed by the wild-type and complemented mutant strains. Biofilms of the luxS mutant exhibited finger-like projections of cells that extended into the flow cell lumen. Thus, the inability to produce AI-2 is associated with altered microcolony architecture within S. gordonii biofilms formed in saliva during a time frame consistent with initial colonization of freshly cleaned enamel surfaces.
Figures
FIG. 1.
(A) Linear map of 6.4 kb of genomic DNA flanking S. gordonii luxS. Predicted ORFs and their orientations are shown. A predicted stem-loop structure between ORF2 and luxS is indicated. (B) Schematic representations of the chromosomal regions encompassing the _ermAM_-disrupted gene of the S. gordonii luxS mutant and the tandem disrupted and intact gene copies of the complemented luxS mutant. The image is not drawn to scale.
FIG. 2.
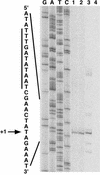
Primer extension mapping of the S. gordonii luxS transcription start site. Labeled cDNA from reverse transcription reactions was run next to luxS sequencing reaction products (lanes G, A, T, and C) generated with the same primer. Reverse transcription products from reaction mixtures containing total RNA isolated from wild-type S. gordonii (lane 1), the complemented luxS mutant (lane 2), E. coli DH5α(pSF151-luxS) (lane 3), and E. coli DH5α without a plasmid (lane 4) are shown. An expanded view of the complementary nucleotide sequence surrounding the transcription start site (+1) is shown, and the putative extended −10 hexamer is highlighted with a vertical bar.
FIG. 3.
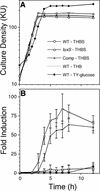
S. gordonii exhibits maximum induction of AI-2 production in THBS. Time courses of culture densities (A) and AI-2 induction levels (B) of wild-type (WT; open circles), luxS mutant (open squares), and complemented (Comp) luxS mutant (open triangles) S. gordonii strains grown in THBS are shown. Culture densities and AI-2 induction levels of the wild-type strain grown in THB lacking serum (dotted lines, no symbols) and in TY-glucose medium (filled diamonds) are also shown. All time course experiments were repeated at least six times, and the averages of three representative independent experiments with standard deviations are shown (B). KU, Klett units.
FIG. 4.
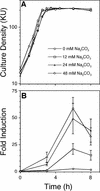
Induction of S. gordonii AI-2 production by Na2CO3 in a concentration-dependent manner. Time courses of culture densities (A) and AI-2 induction levels (B) of wild-type S. gordonii grown in BHIS supplemented with 0 mM (circles), 12 mM (squares), 24 mM (triangles), and 48 mM (diamonds) Na2CO3. All time course experiments were repeated at least six times, and the averages of three representative independent experiments with standard deviations are shown (B). KU, Klett units.
FIG. 5.
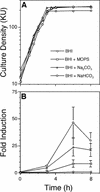
Contribution of carbonate ion to S. gordonii AI-2 production. Time courses of culture densities (A) and AI-2 induction levels (B) of wild-type S. gordonii grown in BHI medium (circles) or BHI medium supplemented with 24 mM MOPS (squares), 24 mM Na2CO3 (triangles), and 24 mM NaHCO3 (diamonds). All time course experiments were repeated at least six times, and the averages of three representative independent experiments with standard deviations are shown (B). KU, Klett units.
FIG. 6.
S. gordonii luxS mutant biofilm phenotype is not apparent in BHI medium. (A) Time course (left to right) of biofilm development in medium-conditioned flow cells (10-fold-diluted BHI medium containing 2.4 mM Na2CO3) by luxS mutant (luxS) and wild-type (WT) S. gordonii strains. _x_-z reconstructions of each biofilm are shown below each _x_-y image. Digital zooms (magnification, 3×) of the lower left corner of each 1-h _x_-y image are shown as insets. (B) Digital zooms (magnification, 3×) of the center of each 1-h _x_-z reconstruction. Scale bars, 50 μm.
FIG. 7.
S. gordonii luxS mutant biofilm phenotype in saliva. (A) Time courses (left to right) of biofilm development in saliva-conditioned flow cells by luxS mutant (luxS), wild-type (WT), and complemented luxS mutant (Comp) S. gordonii strains. _x_-z reconstructions of each biofilm are shown below each _x_-y image. Digital zooms (magnification, 3×) of the lower left corner of each 1-h _x_-y image are shown as insets. (B) Digital zooms (magnification, 3×) of the center of each 1-h _x_-z reconstruction. Scale bars, 50 μm.
Similar articles
- Streptococcus gordonii LuxS/autoinducer-2 quorum-sensing system modulates the dual-species biofilm formation with Streptococcus mutans.
Wang X, Li X, Ling J. Wang X, et al. J Basic Microbiol. 2017 Jul;57(7):605-616. doi: 10.1002/jobm.201700010. Epub 2017 May 9. J Basic Microbiol. 2017. PMID: 28485524 - LuxS-based signaling in Streptococcus gordonii: autoinducer 2 controls carbohydrate metabolism and biofilm formation with Porphyromonas gingivalis.
McNab R, Ford SK, El-Sabaeny A, Barbieri B, Cook GS, Lamont RJ. McNab R, et al. J Bacteriol. 2003 Jan;185(1):274-84. doi: 10.1128/JB.185.1.274-284.2003. J Bacteriol. 2003. PMID: 12486064 Free PMC article. - Autoinducer-2 influences interactions amongst pioneer colonizing streptococci in oral biofilms.
Cuadra-Saenz G, Rao DL, Underwood AJ, Belapure SA, Campagna SR, Sun Z, Tammariello S, Rickard AH. Cuadra-Saenz G, et al. Microbiology (Reading). 2012 Jul;158(Pt 7):1783-1795. doi: 10.1099/mic.0.057182-0. Epub 2012 Apr 5. Microbiology (Reading). 2012. PMID: 22493304 Free PMC article. - The LuxS/AI-2 system of Streptococcus suis.
Wang Y, Wang Y, Sun L, Grenier D, Yi L. Wang Y, et al. Appl Microbiol Biotechnol. 2018 Sep;102(17):7231-7238. doi: 10.1007/s00253-018-9170-7. Epub 2018 Jun 25. Appl Microbiol Biotechnol. 2018. PMID: 29938319 Review. - Functional diversity of AI-2/LuxS system in lactic acid bacteria: Impacts on biofilm formation and environmental resilience.
Kanthenga HT, Banicod RJS, Ntege W, Njiru MN, Javaid A, Tabassum N, Kim YM, Khan F. Kanthenga HT, et al. Res Microbiol. 2025 Mar 22:104296. doi: 10.1016/j.resmic.2025.104296. Online ahead of print. Res Microbiol. 2025. PMID: 40122434 Review.
Cited by
- Integration of Metabolic and Quorum Sensing Signals Governing the Decision to Cooperate in a Bacterial Social Trait.
Boyle KE, Monaco H, van Ditmarsch D, Deforet M, Xavier JB. Boyle KE, et al. PLoS Comput Biol. 2015 Jun 23;11(5):e1004279. doi: 10.1371/journal.pcbi.1004279. eCollection 2015 May. PLoS Comput Biol. 2015. PMID: 26102206 Free PMC article. - Pleiotropic role of quorum-sensing autoinducer 2 in Photorhabdus luminescens.
Krin E, Chakroun N, Turlin E, Givaudan A, Gaboriau F, Bonne I, Rousselle JC, Frangeul L, Lacroix C, Hullo MF, Marisa L, Danchin A, Derzelle S. Krin E, et al. Appl Environ Microbiol. 2006 Oct;72(10):6439-51. doi: 10.1128/AEM.00398-06. Appl Environ Microbiol. 2006. PMID: 17021191 Free PMC article. - Investigation of supragingival plaque microbiota in different caries status of Chinese preschool children by denaturing gradient gel electrophoresis.
Jiang W, Jiang Y, Li C, Liang J. Jiang W, et al. Microb Ecol. 2011 Feb;61(2):342-52. doi: 10.1007/s00248-010-9753-z. Epub 2010 Oct 7. Microb Ecol. 2011. PMID: 20927511 - Molecular characterization and transcription of the luxS gene that encodes LuxS autoinducer 2 synthase in Streptococcus bovis.
Asanuma N, Yoshii T, Hino T. Asanuma N, et al. Curr Microbiol. 2004 Nov;49(5):366-71. doi: 10.1007/s00284-004-4356-x. Curr Microbiol. 2004. PMID: 15486712 - Promoter and transcription analysis of penicillin-binding protein genes in Streptococcus gordonii.
Haenni M, Moreillon P, Lazarevic V. Haenni M, et al. Antimicrob Agents Chemother. 2007 Aug;51(8):2774-83. doi: 10.1128/AAC.01127-06. Epub 2007 May 14. Antimicrob Agents Chemother. 2007. PMID: 17502405 Free PMC article.
References
- Aravind, L., and E. V. Koonin. 1998. The HD domain defines a new superfamily of metal-dependent phosphohydrolases. Trends Biochem. Sci. 23:469-472. - PubMed
- Bardow, A., B. Madsen, and B. Nauntofte. 2000. The bicarbonate concentration in human saliva does not exceed the plasma level under normal physiological conditions. Clin. Oral Investig. 4:245-253. - PubMed
- Bassler, B. L., M. Wright, R. E. Showalter, and M. R. Silverman. 1993. Intercellular signalling in Vibrio harveyi: sequence and function of genes regulating expression of luminescence. Mol. Microbiol. 9:773-786. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources