Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall - PubMed (original) (raw)
. 2003 Aug 1;17(15):1835-40.
doi: 10.1101/gad.266803.
Holger Gerhardt, Stefan Liebner, Alexandra Abramsson, Maria Enge, Mats Hellstrom, Gudrun Backstrom, Simon Fredriksson, Ulf Landegren, Henrik C Nystrom, Goran Bergstrom, Elisabetta Dejana, Arne Ostman, Per Lindahl, Christer Betsholtz
Affiliations
- PMID: 12897053
- PMCID: PMC196228
- DOI: 10.1101/gad.266803
Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall
Per Lindblom et al. Genes Dev. 2003.
Abstract
Several platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF) family members display C-terminal protein motifs that confer retention of the secreted factors within the pericellular space. To address the role of PDGF-B retention in vivo, we deleted the retention motif by gene targeting in mice. This resulted in defective investment of pericytes in the microvessel wall and delayed formation of the renal glomerulus mesangium. Long-term effects of lack of PDGF-B retention included severe retinal deterioration, glomerulosclerosis, and proteinuria. We conclude that retention of PDGF-B in microvessels is essential for proper recruitment and organization of pericytes and for renal and retinal function in adult mice.
Figures
Figure 1
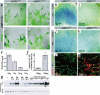
The pdgf-b_ret allele. (a) Outline of the_pdgf-b locus, targeting construct, and Southern identification of the_pdgf-b_ret allele. (b) Schematic outline of the_pdgf-b_ret allele. Remaining loxP site and inserted TAA-stop denoted by arrowhead and asterisk, respectively. (c) C-terminal sequence of the predicted PDGF-Bwt and PDGF-Bret proteins. (d) Comparison of the retention motifs of mouse PDGF and VEGF members. Basic residues in bold. (e) Northern blot analysis of_pdgf-b_ transcripts in pdgf-b+/+,_pdgf-b_ret/+, and _pdgf-b_ret/ret brains. (Left) EtBr-stained gel.
Figure 2.
Expression of the _pdgf-b_ret allele, activity of the PDGF-Bret protein, and deficient recruitment of pericytes in_pdgf-b_ret/ret mice. (_a_-d) In situ hybridization localizes pdgf-b mRNA to endothelial tip cells (arrows). (e,f) PDGF-B protein levels by proximity ligation assay in medium (M) and lysates (L) of wild-type (Wt) and_pdgf-b_ret/ret (Ret) endothelioma cell lines. Triplicate measurements are shown with standard deviations. (g) PDGFR-β phosphorylation induced by the indicated dilutions (%) of 10× concentrated media from endothelioma cells. Western blots with phospho-tyrosine (P-tyr) and PDGFR-β (Rec-β) antibodies are shown. (h_-k) XlacZ4 staining of forebrain from E15.5_pdgf-b+/+, _pdgf-b_ret/+,_pdgf-b_ret/ret, and _pdgf-b_ret/- mice. LacZ-positive pericytes and vSMCs align cerebral/meningeal vessels. (l,m) GFAP (red) and isolectin (green) staining of P5 vibratome-sectioned brain. Up-regulation of GFAP in astrocytes is seen in focal regions of the _pdgf-b_ret/ret brain.
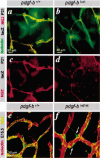
Figure 3.
Defective investment of pericytes into the vessel wall in_pdgf-b_ret/ret mice. (_a_-d) Isolectin (green), NG2 (red), and LacZ stain of pdgf-b+/+ and_pdgf-b_lox/- P21 retinas. The few pericytes seen in_pdgfb_lox/- mice extend thin endothelium-associated processes. (e,f) NG2 and isolectin staining of E12.5 hindbrain. Pericytes (green) are partially detached and extend processes away from the endothelial cells (red; arrows) in _pdgf-b_ret/ret mice.
Figure 4.
Glomerulosclerosis, proteinuria, retinopathy, and vSMC deficiency in_pdgf-b_ret/ret mice. H&E staining shows lack of mesangial cells in _pdgf-b_ret/ret mice at E17.5 (a,b), normal histology at P30 (c,d), and glomerulosclerosis, by PAS staining at P180 (e,f). (g) Albuminuria in _pdgf-b_ret/ret mice at 3 mo of age. (_h_-p) Analysis of postnatal retinas (P7-P180). (_h_-i) Retinal disorganization in_pdgf-b_ret/ret mice, with fibrosis and invasion of RPE (arrow). (j,k) Isolectin (red) and SMA (green) staining of flat-mounted mouse retinas. Arrowhead indicates area of SMA-positive cells outside vessel walls. (l,m) Different density and organization of arterial SMCs in pdgf-b+/+ and_pdgf-b_ret/ret retinas. (n,o) XlacZ4 staining of P30 retinas shows defective pericyte recruitment in_pdgf-b_ret/ret mice and formation of vSMC sheets (arrowheads). (o) Arrows point at residual vessel-associated pericytes in peripheral regions. (p) Retina of P7_pdgf-b_ret/ret mouse with SMA-positive pericyte partially detached from the endothelium. ON, optic nerve; OD, optic disc.
Figure 5.
Retinal vascular development in _pdgf-b_ret/ret mice. Retinal vessels in control (_pdgf-b_ret/+) and_pdgf-b_ret/ret mice at P5. EC, isolectin (green); pericytes, NG2 (red); pericyte nuclei, XlacZ4 (dark blue, arrows). Peripheral (a,b) and central (e,f) regions in_pdgf-b_ret/+ mice show formation of regular vascular plexuses. Note extensive coverage with pericytes (b,f), except for sprouting tips (b). In _pdgfb_ret/ret mice, plexus spreading is delayed (c; peripheral), and irregular (g; central) with regions of hyperfusion (g, arrow), and reduced pericyte density (d,h). Peripheral (_i_-l) and central (_m_-p) regions at high magnification show that peripheral sprouting is sparse in _pdgfb_ret/ret mice, leading to a wide-meshed, irregular vasculature partially devoid of pericytes. Few pericytes are present on remodeling arteries (cf. n and p, arrows).
Similar articles
- Platelet-derived growth factor-B enhances glioma angiogenesis by stimulating vascular endothelial growth factor expression in tumor endothelia and by promoting pericyte recruitment.
Guo P, Hu B, Gu W, Xu L, Wang D, Huang HJ, Cavenee WK, Cheng SY. Guo P, et al. Am J Pathol. 2003 Apr;162(4):1083-93. doi: 10.1016/S0002-9440(10)63905-3. Am J Pathol. 2003. PMID: 12651601 Free PMC article. - Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury.
Yonekura H, Yamamoto Y, Sakurai S, Petrova RG, Abedin MJ, Li H, Yasui K, Takeuchi M, Makita Z, Takasawa S, Okamoto H, Watanabe T, Yamamoto H. Yonekura H, et al. Biochem J. 2003 Mar 15;370(Pt 3):1097-109. doi: 10.1042/BJ20021371. Biochem J. 2003. PMID: 12495433 Free PMC article. - Structural and functional specificities of PDGF-C and PDGF-D, the novel members of the platelet-derived growth factors family.
Reigstad LJ, Varhaug JE, Lillehaug JR. Reigstad LJ, et al. FEBS J. 2005 Nov;272(22):5723-41. doi: 10.1111/j.1742-4658.2005.04989.x. FEBS J. 2005. PMID: 16279938 Review.
Cited by
- Targeting TREM2 signaling shows limited impact on cerebrovascular calcification.
Sridhar S, Zhou Y, Ibrahim A, Bertazzo S, Wyss T, Swain A, Maheshwari U, Huang SF, Colonna M, Keller A. Sridhar S, et al. Life Sci Alliance. 2024 Oct 28;8(1):e202402796. doi: 10.26508/lsa.202402796. Print 2025 Jan. Life Sci Alliance. 2024. PMID: 39467636 Free PMC article. - The Role of Pericytes in Inner Ear Disorders: A Comprehensive Review.
Maniaci A, Briglia M, Allia F, Montalbano G, Romano GL, Zaouali MA, H'mida D, Gagliano C, Malaguarnera R, Lentini M, Graziano ACE, Giurdanella G. Maniaci A, et al. Biology (Basel). 2024 Oct 8;13(10):802. doi: 10.3390/biology13100802. Biology (Basel). 2024. PMID: 39452111 Free PMC article. Review. - Human vascularized macrophage-islet organoids to model immune-mediated pancreatic β cell pyroptosis upon viral infection.
Yang L, Han Y, Zhang T, Dong X, Ge J, Roy A, Zhu J, Lu T, Jeya Vandana J, de Silva N, Robertson CC, Xiang JZ, Pan C, Sun Y, Que J, Evans T, Liu C, Wang W, Naji A, Parker SCJ, Schwartz RE, Chen S. Yang L, et al. Cell Stem Cell. 2024 Aug 30:S1934-5909(24)00293-5. doi: 10.1016/j.stem.2024.08.007. Online ahead of print. Cell Stem Cell. 2024. PMID: 39232561 - Macrophages upregulate mural cell-like markers and support healing of ischemic injury by adopting functions important for vascular support.
Amoedo-Leite C, Parv K, Testini C, Herrera-Hidalgo C, Xu F, Giraud A, Malaquias M, Fasterius E, Holl D, Seignez C, Göritz C, Christoffersson G, Phillipson M. Amoedo-Leite C, et al. Nat Cardiovasc Res. 2024 Jun;3(6):685-700. doi: 10.1038/s44161-024-00478-0. Epub 2024 Jun 6. Nat Cardiovasc Res. 2024. PMID: 39196227 Free PMC article. - Human Vascularized Macrophage-Islet Organoids to Model Immune-Mediated Pancreatic β cell Pyroptosis upon Viral Infection.
Yang L, Han Y, Zhang T, Dong X, Ge J, Roy A, Zhu J, Lu T, Vandana JJ, de Silva N, Robertson CC, Xiang JZ, Pan C, Sun Y, Que J, Evans T, Liu C, Wang W, Naji A, Parker SCJ, Schwartz RE, Chen S. Yang L, et al. bioRxiv [Preprint]. 2024 Aug 6:2024.08.05.606734. doi: 10.1101/2024.08.05.606734. bioRxiv. 2024. PMID: 39149298 Free PMC article. Updated. Preprint.
References
- Allavena P., Dejana, E., Bussolino, F., Vecchi, A., and Mantovani, A. 1995. Cytokine regulation of endothelial cells. In Cytokines: A practical approach (ed. F.R. Balkwill), pp. 225-245. IRL Press, Oxford.
- Allt G. and Lawrenson, J.G. 2001. Pericytes: Cell biology and pathology. Cells Tissues Organs 169: 1-11. - PubMed
- Andersson M., Ostman, A., Westermark, B., and Heldin, C.H. 1994. Characterization of the retention motif in the C-terminal part of the long splice form of platelet-derived growth factor A-chain. J. Biol. Chem. 269: 926-9330. - PubMed
- Baeg G.H. and Perrimon, N. 2000. Functional binding of secreted molecules to heparin sulphate proteoglycans in Drosophila. Curr. Opin. Cell Biol. 12: 575-580. - PubMed
- Bussolino F., De Rossi, M., Sica, A., Colotta, F., Wang, J.M., Bocchietto, E., Padura, I.M., Bosia, A., Dejana, E., and Mantovani, A. 1991. Murine endothelioma cell lines transformed by polyoma middle T oncogene as target for and producers of cytokines. J. Immunol. 147: 2122-2129. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases