The N and C termini of the splice variants of the human mitogen-activated protein kinase-interacting kinase Mnk2 determine activity and localization - PubMed (original) (raw)
The N and C termini of the splice variants of the human mitogen-activated protein kinase-interacting kinase Mnk2 determine activity and localization
Gert C Scheper et al. Mol Cell Biol. 2003 Aug.
Abstract
The cap-binding eukaryotic initiation factor eIF4E is phosphorylated by the mitogen-activated protein (MAP) kinase-interacting kinases (Mnk's). Three forms of the Mnk's exist in human cells: Mnk1, Mnk2a, and Mnk2b. These last two are derived from the same gene by alternative splicing and differ only at their C termini. While Mnk2a contains a MAP kinase-binding site in this region, Mnk2b lacks such a sequence and is much less readily activated by MAP kinases in vitro. Expression of Mnk2b in mammalian cells leads to increased phosphorylation of eIF4E, showing that it acts as an eIF4E kinase in vivo. While Mnk2a is cytoplasmic, a substantial amount of Mnk2b is found in the nucleus. Both enzymes contain a stretch of basic residues in their N termini that plays a role in binding to eIF4G and functions as a nuclear localization signal. Binding of eIF4G or nuclear import appears to be regulated by the C terminus of Mnk2a. Furthermore, the MAP kinase-binding site of Mnk2a regulates nuclear entry. Within the nucleus, Mnk2b and certain variants of Mnk2a that are present in the nucleus colocalize with the promyelocytic leukemia protein PML, which also binds to eIF4E.
Figures
FIG. 1.
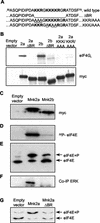
Schematic representation of the Mnk's. (A) Derivation of the mRNAs for Mnk2a and Mnk2b. Human Mnk2b is a splice variant that differs from human Mnk2b only in its C-terminal exon. The 12 exons that are shared by Mnk2a and Mnk2b, and the 13th exon, which is unique in each isoform, are indicated by solid rectangles. Numbering above the diagram refers to EMBL sequence AC007136. (B) Domains in Mnk1, Mnk2a, and Mnk2b. The different domains are discussed in the text. The human forms of Mnk2 contain an N-terminal extension (light grey striped area) of unknown function. All three Mnk's contain a basic region that has been proposed to be important in eIF4G binding (49), but this region could also contain an NLS. All variants contain a catalytic domain with conserved threonyl residues (T) in the activation domain that need to be phosphorylated (P) for activity. Mnk1 contains a consensus sequence for CRM-1-mediated nuclear export (NES). As shown in Fig. 6, one of the critical residues is not present in Mnk2a. Approximately 10 to 20 residues from the C termini of Mnk1 and Mnk2a contain a binding site for MAP kinases; the sequences for this binding site are given, with residues that are conserved among several MAP kinase substrates shown in boldface.
FIG. 2.
Mnk2a and Mnk2b can both phosphorylate eIF4E. (A) Amino acid sequences of residues 50 to 75 of human Mnk2a and Mnk2b. The basic region is boldfaced. The deletion of the whole region (in ΔBR) and the triple Ala mutations made in this study (KKR/AAA and KKK/AAA) are underlined. (B) The basic region is involved in eIF4G binding. HEK293 cells were transfected with the indicated constructs (the empty vector is pCS3MT), and after harvesting of the cells, Myc-tagged proteins were immunoprecipitated. Aliquots of the purified proteins were analyzed directly by Western blotting to determine expression levels (lower panel), and coimmunoprecipitated eIF4G was detected with antibodies directed against the C terminus of eIF4G (22). 2a, Mnk2a; 2b, Mnk2b. Both ΔBR constructs lack the sequence KKRGKKKKR (residues 60 to 68) (upper panel). (C) Expression of Mnk2a and Mnk2b. HEK293 cells were transiently transfected with the indicated pCS3MT constructs, and the cells were harvested as described in Materials and Methods. The Myc-tagged proteins were immunoprecipitated with anti-Myc antibodies, and the levels of expression were analyzed by Western blotting. (D) The immunoprecipitated kinases, as shown in panel B, were tested in vitro in the presence of [γ-32P]ATP for their abilities to phosphorylate recombinant eIF4E as described in Materials and Methods. The part of the autoradiogram showing labeled eIF4E is shown. (E) In vivo activitiesof human Mnk2a and human Mnk2b were assessed by purification of the endogenous eIF4E from extracts of the transfected cells and separation of the phosphorylated (eIF4E+P) and nonphosphorylated (eIF4E) forms by one-dimensional isoelectric focusing. (F) Coimmunoprecipitation of ERK was detected by probing the blot used in panel A with ERK-specific antibodies. (G) Binding of Mnk2a to eIF4G enhances eIF4E phosphorylation. HEK293 cells were transfected with an empty vector (pCS3MT) or vectors encoding Myc-tagged wild-type Mnk2a or Mnk2a with the basic region deleted. After harvesting of the cells, the phosphorylation state of endogenous eIF4E was analyzed by isoelectric focusing as described in Materials and Methods. Annotation is as for panel E.
FIG. 3.
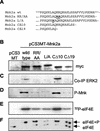
Regulation of Mnk2 activity. (A) Mnk2a is a much better substrate for MAP kinase in vitro than Mnk2b. Equal amounts (2 μg) of _E. coli_-expressed recombinant GST-Mnk2a (top panel) and GST-Mnk2b (bottom panels) were incubated with 6 mU of recombinant active p38 MAP kinase (kindly provided by the Division of Signal Transduction Therapy, University of Dundee) in a volume of 20 μl for the indicated times. The kinase reactions were then stopped by addition of SDS-PAGE sample buffer and heating of the sample at 95°C for 5 min. Phosphorylation of the threonines in the activation domain of the Mnk's was analyzed by Western blotting with phosphospecific antibodies (from Cell Signaling Technology). Two exposures are shown for Mnk2b, one of 20 s to allow direct comparison to the upper panel showing phosphorylation of Mnk2a, and one of 5 min to visualize phosphorylation of Mnk2b at earlier time points. The figure is an immunoblot. (B) In vitro-phosphorylated recombinant Mnk2b phosphorylates Ser209. Recombinant wild-type eIF4E or eIF4E(S209A) was incubated with recombinant Mnk2b that was activated as described above in the presence or absence of SB203580 as indicated below the gel. As a control the same amount of p38 MAPKα that was used to activate Mnk2b was also incubated with recombinant eIF4E. (C) Analysis of Mnk2a and Mnk2b activities. Recombinant GST-Mnk2a and GST-Mnk2b were phosphorylated as described above for 1 h; then the activity of p38 MAPKα was inhibited by addition of SB203580, and the activated Mnk's were incubated with recombinant eIF4E and [γ-32P]ATP for the indicated times. Phosphorylation of eIF4E was visualized by SDS-PAGE and autoradiography. (D) Expression of Myc-tagged Mnk1, Mnk2a, and Mnk2b. pCS3MT constructs encoding fusion proteins of Mnk1, Mnk2a, or Mnk2b were expressed in transfected HEK293 cells, and after the cells were harvested, the proteins were immunoprecipitated with antibodies against the Myc tag. Western blotting showed very similar expression levels of these proteins under three different conditions: −FCS, TPA, and PD SB. (i) −FCS (without fetal calf serum), after 26 h of transfection the growth medium was replaced byserum-free medium and cells were grown for an additional 16 h. (ii) TPA, cells were treated as described above, but 30 min before harvesting, they were stimulated with 500 μM TPA for 30 min. (iii) PD SB, cells were treated as described for the starved cells followed by incubation with 10 μM PD98059 and 50 μM SB203580 for 4 h before harvesting. These three conditions are indicated under panel H and apply to panels D through H. (E) Stimulation of the ERK pathway by TPA. The phosphorylation of Erk (P-Erk) was analyzed by Western blotting of the cell extracts with phosphospecific anti-Erk antibodies. (F) eIF4E kinase activity. Kinases were incubated with recombinant eIF4E and [γ-32P]ATP, and phosphorylation of eIF4E was visualized by SDS-PAGE and autoradiography. Numbers below the lanes are relative activities (as determined by using a phosphorimager [Fuji]) compared to that under the control condition (−FCS) for each separate kinase. (G) Phosphorylation of the Mnk's. The phosphorylation states of the three Mnk's under the different conditions were analyzed by Western blotting with antibodies directed against the phosphorylated threonines in the activation domain. (H) Assessment of in vivo kinase activity. The activities of the kinases were assessed by purification of endogenous eIF4E from the cell extracts by m7GTP-Sepharose chromatography, isoelectric focusing. and Western blotting with antibodies against eIF4E. The leftmost lane shows the level of eIF4E phosphorylation in untransfected control (C) cells. Numbers below the gel represent the percentage of eIF4E in its phosphorylated form, as determined by quantification of the data using ImageJ software (
).
FIG. 4.
C-terminal mutations affect Mnk2a activity. (A) C-terminal sequence of human Mnk2a (residues 429 to 465) and the sequences of four variants used in these studies. In the MAP kinase-binding mutants RR/AA and L/A, the changed residues are underlined and boldfaced. (B) Loss of Erk binding reduces activity. The indicated variants were expressed from pCS3MT constructs (with pCS3MT as the empty vector control) in HEK293 cells, and Myc-tagged proteins were immunoprecipitated with anti-Myc antibodies. Western blotting with anti-Myc antibodies shows very similar expression levels for all variants. The L/A variant migrates somewhat more slowly, as it was cloned from GFP-Mnk2a(RR/AA) into the _Eco_RI site of pCS3MT, instead of the _Nco_I site, adding 15 amino acids between the Myc tag of pCS3MT and the first methionine of Mnk1. (C) Bound Erk was analyzed by probing the same blot with anti-Erk. (D) Aliquots identical to those used for panels B and C were run on a different gel, and samples were analyzed by Western blotting with phosphospecific anti-Mnk antibodies. (E) The Myc immunoprecipitates were incubated with recombinant eIF4E and [γ-32P]ATP, and phosphorylation of eIF4E was visualized by SDS-PAGE and autoradiography. (F) Endogenous eIF4E from the cell extracts used in panels B through E was purified by m7GTP-Sepharose chromatography. Phosphorylated and nonphosphorylated forms of eIF4E (as indicated on the right) were separated by isoelectric focusing and visualized by Western blotting with antibodies against eIF4E.
FIG. 5.
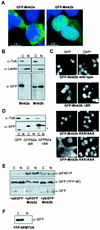
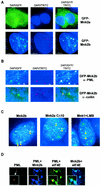
Differential localization of Mnk2a and Mnk2b. (A) Mnk2b is found in the nucleus. HEK293 cells were grown on coverslips and transfected with constructs encoding GFP-tagged versions of Mnk2a or Mnk2b. After fixation of the cells with paraformaldehyde and treatment with DAPI, the presence of fluorescent proteins in the cytoplasm and nucleus was visualized as described in Materials and Methods. An overlay of immunofluorescence (green) and DNA staining in the nucleus (blue) is shown. (B) Extracts from transfected cells were fractionated to give cytoplasmic (C) and nuclear (N) fractions as described in Materials and Methods. Aliquots representing 10% of each fraction were analyzed by Western blotting with antibodies directed againstGFP, lamin B, and α-tubulin. (C) The first part of the basic region is not required for nuclear localization of Mnk2b. GFP-tagged versions of wild-type Mnk2b or the indicated variants were expressed in HEK293 cells grown on coverslips. Cells were treated as described above. Separate images for immunofluorescence (GFP) and nuclear staining (DAPI) are shown. Arrowheads in the righthand panels indicate nuclei of transfected cells. (D) The N terminus of human Mnk2 functions as an NLS. HEK293 cells were transfected with constructs expressing GFP or GFP fusions with the N terminus of human Mnk2 up to residue 60 (−BR) or up to residue 70 (+BR). Cells were harvested in EZ lysis buffer, and cytoplasmic and nuclear extracts were prepared as described in Materials and Methods. The distribution of the GFP-tagged proteins was visualized by Western blotting with anti-GFP. The asterisk indicates a product which is most likely derived from an N-terminal truncation, as similar bands are found for GFP alone or GFP fused to the N terminus of human Mnk2a up to residue 60. (E) Expression of Mnk2a or Mnk2b results in phosphorylation of eIF4E in the cytoplasm and nucleus. HEK293 cells were transfected with 5 μg of pEYFP-eIF4E in combination with 5 μg of pEGFP-C1, pEGFP-Mnk2a, or pEGFP-Mnk2b as indicated. After 24 h of transfection, cells were harvested in EZ lysis buffer, and extracts were fractionated into cytoplasmic (C) and nuclear (N) fractions. Aliquots of these fractions representing, respectively, 8 and 15% of the total were analyzed for the presence of phosphorylated eIF4E by Western blotting with a phosphospecific anti-eIF4E antibody (a generous gift from Simon Morley, University of Sussex) (top panel). Equal samples were analyzed by using anti-GFP as a control for the loading of the total amount of YFP-tagged eIF4E (center panel) and for the presence of the GFP-tagged proteins in the cytoplasmic and nuclear fractions (bottom panel). It should be noted that the part of the figure showing GFP was aligned with the GFP-Mnk's. This lefthand part was derived from the same film as the righthand part. (F) HEK293 cells were transfected with a construct encoding YFP-tagged eIF4E in which Trp73 was replaced by alanine (W73A). Cytoplasmic and nuclear fractions were made as described above, and the presence of the YFP-tagged protein was analyzed by Western blotting with antibodies against GFP.
FIG. 6.
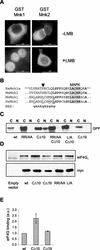
The C terminus of Mnk2a is involved in localization. (A) The localization of Mnk2 is not sensitive to leptomycin B. Mouse Mnk1 and Mnk2 were expressed as GST fusion proteins in HEK293 cells grown on coverslips. After 24 h, cells were either left untreated or incubated in the presence of 5 ng of leptomycin B (LMB)/ml for 4 h. Cells were then fixed with paraformaldehyde and permeabilized, and GST fusion proteins were visualized by using anti-GST monoclonal antibodies and FITC-conjugated anti-mouse secondary antibodies. (B) Alignment of residues 425 to 451 of human Mnk2a with corresponding regions of other Mnk's. In mouse and human Mnk1, a consensus sequence (underlined residues) can be found that could func-tion as an NES (bottom sequence, in which ψ represents a hydrophobic residue and X represents any amino acid). The solid arrowhead indicates a serine in the Mnk2 forms that would likely abolish NES function. Fully conserved amino acids are boldfaced, and the MAP kinase-binding site is shaded. (C) GFP fusion proteins of the variants indicated (see also Fig. 4) were expressed in transfected HEK293 cells. After being harvested in EZ lysis buffer, cell extracts were fractionated into cytoplasmic (C) and nuclear (N) fractions. The presence of the various forms of Mnk2a was revealed by Western blotting with antibodies against GFP. (D) C-terminal deletions affect binding of eIF4G to Mnk2a. HEK293 cells were transfected with an empty vector (pCS3MT) or vectors encoding Myc-tagged wild-type Mnk2a or Mnk2a variants as indicated. After harvesting of the cells, the expressed proteins were immunoprecipitated with antibodies against the Myc tag. Coimmunoprecipitated eIF4G was visualized by Western blotting. (E) Quantification of eIF4G binding. The signals for eIF4G and Mnk2 obtained in three similar experiments, as shown in panel D, were quantified with ImageJ software, and the relative binding of eIF4G to wild-type Mnk2a or the indicated variants was determined. Means and standard deviations are shown. The binding of eIF4G to wild-type Mnk2a was arbitrarily set at 1.
FIG. 7.
Mnk2b colocalizes with PML. (A) HEK293 cells grown on coverslips were transfected with the indicated constructs. After incubation with antibodies against PML, microscopic images were recorded as described in Materials and Methods. DAPI indicates DAPI staining of the nuclei, GFP indicates fluorescence microscopy to detect GFP-tagged proteins, and TRITC indicates the use of TRITC-conjugated secondary antibodies to detect PML. (B) Colocalization of GFP-Mnk2b with PML and not with coilin. Coverslips with GFP-Mnk2b-transfected cells were incubated with an anti-PML or anti-coilin antibody and with TRITC-conjugated secondary antibodies as indicated. An enlargement of the nucleus showing Mnk2b-PML speckles is shown. (C) HEK293 cells grown on coverslips were transfected with GFP-Mnk2b, GFP-Mnk2aCΔ10, or GFP-Mnk1. In the last case, the transfected cells were treated with leptomycin B (LMB) (5 ng/ml) for 4 h before fixation. Colocalization with PMLwas determined as described in Materials and Methods. Nuclei of the transfected cells were visualized by staining with DAPI (blue). Yellow arrowheads indicate some of the bodies in which PML and the GFP-tagged kinases colocalize. (D) Colocalization of Mnk2b, eIF4E, and PML. HEK293 cells were transfected with constructs expressing CFP-tagged Mnk2b and YFP-tagged eIF4E. The latter construct contains an additional sequence corresponding to the simian virus 40 T-antigen NLS in order to increase the levels of eIF4E in the nucleus. CFP and YFP intensities were very similar in these experiments. After fixation and permeabilization, PML was visualized by immunofluorescence as described in Materials and Methods. DeltaVision microscopy images of nuclei were recorded using the filters for TRITC (PML), TRITC and CFP (PML + Mnk2b), TRITC and YFP (PML + eIF4E), and CFP and YFP (Mnk2b + eIF4E). These four images were taken from the same Z-plane. Insets in each panel show a part of the image in greater detail.
FIG. 8.
Involvement of the N and C termini in the regulation of Mnk2. (A) Schematic presentation of Mnk2a bound to MAP kinase (MAPK). The basic region is shown with two different patterns to indicate that the NLS and the eIF4G-binding site overlap but are not identical. Limited binding of importin α and eIF4G can occur in the wild-type Mnk2a, as indicated by the dashed arrow. (B) Schematic presentation of Mnk2b. The shorter C-terminal region of Mnk2b does not interfere with access to the N terminus, and therefore Mnk2b can bind better to importin α and eIF4G (as indicated by the solid arrow). (C) Possible explanation of the effects of changes in the C terminus of Mnk2a. In Mnk2aCΔ10 the interaction between the N and C termini is reduced, allowing a conformational change dependent on binding to MAPK. This results in increased access for importin α and eIF4G. Due to the lack of a MAPK-binding site in the CΔ19 or RR/AA (or L/A) variant, this conformational change cannot occur and the basic region is less accessible.
Similar articles
- Features in the N and C termini of the MAPK-interacting kinase Mnk1 mediate its nucleocytoplasmic shuttling.
Parra-Palau JL, Scheper GC, Wilson ML, Proud CG. Parra-Palau JL, et al. J Biol Chem. 2003 Nov 7;278(45):44197-204. doi: 10.1074/jbc.M302398200. Epub 2003 Aug 28. J Biol Chem. 2003. PMID: 12949082 - Regulation of eukaryotic initiation factor 4E (eIF4E) phosphorylation by mitogen-activated protein kinase occurs through modulation of Mnk1-eIF4G interaction.
Shveygert M, Kaiser C, Bradrick SS, Gromeier M. Shveygert M, et al. Mol Cell Biol. 2010 Nov;30(21):5160-7. doi: 10.1128/MCB.00448-10. Epub 2010 Sep 7. Mol Cell Biol. 2010. PMID: 20823271 Free PMC article. - The mitogen-activated protein kinase signal-integrating kinase Mnk2 is a eukaryotic initiation factor 4E kinase with high levels of basal activity in mammalian cells.
Scheper GC, Morrice NA, Kleijn M, Proud CG. Scheper GC, et al. Mol Cell Biol. 2001 Feb;21(3):743-54. doi: 10.1128/MCB.21.3.743-754.2001. Mol Cell Biol. 2001. PMID: 11154262 Free PMC article. - The Mnks: MAP kinase-interacting kinases (MAP kinase signal-integrating kinases).
Buxade M, Parra-Palau JL, Proud CG. Buxade M, et al. Front Biosci. 2008 May 1;13:5359-73. doi: 10.2741/3086. Front Biosci. 2008. PMID: 18508592 Review. - Phosphorylation of the cap-binding protein eIF4E by the MAPK-activated protein kinase Mnk1.
Pyronnet S. Pyronnet S. Biochem Pharmacol. 2000 Oct 15;60(8):1237-43. doi: 10.1016/s0006-2952(00)00429-9. Biochem Pharmacol. 2000. PMID: 11007962 Review.
Cited by
- Targeted splicing therapy: new strategies for colorectal cancer.
Zheng Y, Zhong G, He C, Li M. Zheng Y, et al. Front Oncol. 2023 Aug 17;13:1222932. doi: 10.3389/fonc.2023.1222932. eCollection 2023. Front Oncol. 2023. PMID: 37664052 Free PMC article. Review. - Pharmacological and genetic evaluation of proposed roles of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase (MEK), extracellular signal-regulated kinase (ERK), and p90(RSK) in the control of mTORC1 protein signaling by phorbol esters.
Fonseca BD, Alain T, Finestone LK, Huang BP, Rolfe M, Jiang T, Yao Z, Hernandez G, Bennett CF, Proud CG. Fonseca BD, et al. J Biol Chem. 2011 Aug 5;286(31):27111-22. doi: 10.1074/jbc.M111.260794. Epub 2011 Jun 9. J Biol Chem. 2011. PMID: 21659537 Free PMC article. - Alternative RNA Splicing-The Trojan Horse of Cancer Cells in Chemotherapy.
Mehterov N, Kazakova M, Sbirkov Y, Vladimirov B, Belev N, Yaneva G, Todorova K, Hayrabedyan S, Sarafian V. Mehterov N, et al. Genes (Basel). 2021 Jul 18;12(7):1085. doi: 10.3390/genes12071085. Genes (Basel). 2021. PMID: 34356101 Free PMC article. Review. - Medicinal chemistry approaches to target the MNK-eIF4E axis in cancer.
Fernandez A, Monsen PJ, Platanias LC, Schiltz GE. Fernandez A, et al. RSC Med Chem. 2023 May 9;14(6):1060-1087. doi: 10.1039/d3md00121k. eCollection 2023 Jun 22. RSC Med Chem. 2023. PMID: 37360400 Free PMC article. Review. - Understanding and Targeting the Eukaryotic Translation Initiation Factor eIF4E in Head and Neck Cancer.
Culjkovic B, Borden KL. Culjkovic B, et al. J Oncol. 2009;2009:981679. doi: 10.1155/2009/981679. Epub 2009 Dec 13. J Oncol. 2009. PMID: 20049173 Free PMC article.
References
- Borden, K. L. 2000. RING domains: master builders of molecular scaffolds? J. Mol. Biol. 295:1103-1112. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous