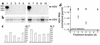In vivo antiviral efficacy of prenylation inhibitors against hepatitis delta virus - PubMed (original) (raw)
. 2003 Aug;112(3):407-14.
doi: 10.1172/JCI17704.
Junko Ohkanda, Ping Liu, So-Young Lee, F H Salazar, Patricia L Marion, Kazuo Ohashi, Leonard Meuse, Mark A Kay, John L Casey, Said M Sebti, Andrew D Hamilton, Jeffrey S Glenn
Affiliations
- PMID: 12897208
- PMCID: PMC166292
- DOI: 10.1172/JCI17704
In vivo antiviral efficacy of prenylation inhibitors against hepatitis delta virus
Bruno B Bordier et al. J Clin Invest. 2003 Aug.
Abstract
Hepatitis delta virus (HDV) can dramatically worsen liver disease in patients coinfected with hepatitis B virus (HBV). No effective medical therapy exists for HDV. The HDV envelope requires HBV surface antigen proteins provided by HBV. Once inside a cell, however, HDV can replicate its genome in the absence of any HBV gene products. In vitro, HDV virion assembly is critically dependent on prenyl lipid modification, or prenylation, of its nucleocapsid-like protein large delta antigen. To overcome limitations of current animal models and to test the hypothesis that pharmacologic prenylation inhibition can prevent the production of HDV virions in vivo, we established a convenient mouse-based model of HDV infection capable of yielding viremia. Such mice were then treated with the prenylation inhibitors FTI-277 and FTI-2153. Both agents were highly effective at clearing HDV viremia. As expected, HDV inhibition exhibited duration-of-treatment dependence. These results provide the first preclinical data supporting the in vivo efficacy of prenylation inhibition as a novel antiviral therapy with potential application to HDV and a wide variety of other viruses.
Figures
Figure 1
Intrahepatic replication of HDV following hydrodynamic transfection. Mice transgenic for HBV were hydrodynamically transfected with vector DNA (pcDNA3) or a vector bearing HDV replication-inducing sequences [pCMV·HDVI(+)]. Seven days later, mice were sacrificed and liver samples were analyzed for HDV RNA and protein. (a) Samples of total liver RNA from a mouse transfected with pcDNA3 (lane 1) or with pCMV·HDVI(+) (lane 2) were analyzed for HDV genomic RNA using Northern blots. Positions of molecular-weight markers are on the left; arrow indicates position of the replicated 1.7-kb RNA genome. (b) Samples of total liver RNA from a mouse transfected with pcDNA3 (lane 1) or with pCMV·HDVI(+) (lane 2) were analyzed for delta antigen using Western blots. Positions of molecular-weight markers are on the left; arrow indicates position of delta antigen (δAg). (c) Representative time course for HDV replication in hydrodynamically transfected mice. Mice transgenic for HBV were hydrodynamically transfected with pcDNA3 (lane 1) or pCMV·HDVI(+) (lanes 2–4) and were sacrificed at day 2 (lane 2), day 4 (lane 3), or day 7 (lanes 1 and 4) after transfection. Liver samples were analyzed for HDV RNA using Northern blots. To account for potential differences in transfection efficiency, a plasmid encoding hAAT was included in the transfection, and HDV replication was detected in mice who had similar serum levels of hAAT on day 2 after transfection. (d and e) Liver sections from the same mice as in a were fixed in formalin and stained by immunohistochemistry for detection of HDV delta antigen. (d) Mouse transfected with pcDNA3. (e) Mouse transfected with pCMV·HDVI(+). Brown-red spots indicate characteristic nuclear staining pattern of delta antigen.
Figure 2
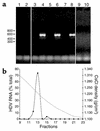
HDV viremia following hydrodynamic transfection of HBV transgenic mice. (a) After hydrodynamic transfection, mice sera were tested for HDV genomic RNA by RT-PCR. Ethidium bromide gel of sera from HBV-transgenic mice transfected with vector alone assayed at day 2 (lane 1) and day 7 (lane 2); sera from three transgenic mice transfected with pCMV·HDVIII(+) and pGEM4ayw.2× assayed at day 2 (lanes 3, 5, and 7) and at day 7 (lanes 4, 6, and 8); sera from a nontransgenic mouse transfected with pCMV·HDVIII(+) and assayed at day 2 (lane 9) and day 7 (lane 10). (b) Aliquots of day-7 sera from mice as analyzed in lanes 4, 6, and 8 of a were banded in a CsCl gradient, and fractions were assayed for HDV RNA by quantitative RT-PCR (filled circles) and CsCl density by refractometry (gray line).
Figure 3
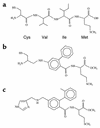
Design and structures of prenylation inhibitors used to treat HDV viremia. (a) Cys-Val-Ile-Met CXXX box tetrapeptide as found in K-Ras4B, a substrate of farnesyltransferase. Two peptidomimetics of this CXXX box were designed as, and shown to be, competitive inhibitors of farnesyltransferase, (b) FTI-277, wherein the dipeptide Val-Ile is replaced by 2-phenyl-4-aminobenzoic acid, and (c) FTI-2153, wherein the tripeptide Cys-Val-Ile is replaced by an imidazole derivative of 2-(2-methylphenyl)-4-aminomethylbenzoic acid.
Figure 4
In vivo treatment of HDV with prenylation inhibitors. (a) Mice hydrodynamically transfected to produce HDV viremia were treated with carrier alone (lanes 1 and 6), carrier plus FTI-277 (lanes 2–5), or carrier plus FTI-2153 (lanes 7–10) for 7 days. Serum aliquots were then assayed for HDV RNA by RT-PCR. (b) Corresponding liver samples were analyzed for HDV RNA using Northern blots. (c) Serum aliquots were also analyzed for ALT. (d) Mice hydrodynamically transfected to produce HDV viremia were treated with carrier alone (controls, filled circles) or carrier plus FTI-2153 (open circles) for the indicated number of days prior to sacrifice. Serum HDV RNA was quantitated by RT-PCR. HDV RNA per microgram of total liver RNA was quantitated by Northern blots and used to normalize for any differences in transfection efficiency. The mean serum HDV RNA genome equivalent (geq) for each group of mice is plotted (see Methods for additional details).
Comment in
- Denying the wolf access to sheep's clothing.
Heller T, Hoofnagle JH. Heller T, et al. J Clin Invest. 2003 Aug;112(3):319-21. doi: 10.1172/JCI19417. J Clin Invest. 2003. PMID: 12897197 Free PMC article.
Similar articles
- Denying the wolf access to sheep's clothing.
Heller T, Hoofnagle JH. Heller T, et al. J Clin Invest. 2003 Aug;112(3):319-21. doi: 10.1172/JCI19417. J Clin Invest. 2003. PMID: 12897197 Free PMC article. - A prenylation inhibitor prevents production of infectious hepatitis delta virus particles.
Bordier BB, Marion PL, Ohashi K, Kay MA, Greenberg HB, Casey JL, Glenn JS. Bordier BB, et al. J Virol. 2002 Oct;76(20):10465-72. doi: 10.1128/jvi.76.20.10465-10472.2002. J Virol. 2002. PMID: 12239323 Free PMC article. - Dynamics of in vivo hepatitis D virus infection.
Goyal A, Murray JM. Goyal A, et al. J Theor Biol. 2016 Jun 7;398:9-19. doi: 10.1016/j.jtbi.2016.03.018. Epub 2016 Mar 21. J Theor Biol. 2016. PMID: 27012516 - Prenylation of HDAg and antiviral drug development.
Glenn JS. Glenn JS. Curr Top Microbiol Immunol. 2006;307:133-49. doi: 10.1007/3-540-29802-9_7. Curr Top Microbiol Immunol. 2006. PMID: 16903224 Review. - Management of hepatitis delta: Need for novel therapeutic options.
Abbas Z, Abbas M. Abbas Z, et al. World J Gastroenterol. 2015 Aug 28;21(32):9461-5. doi: 10.3748/wjg.v21.i32.9461. World J Gastroenterol. 2015. PMID: 26327754 Free PMC article. Review.
Cited by
- What role for cellular metabolism in the control of hepatitis viruses?
Diaz O, Vidalain PO, Ramière C, Lotteau V, Perrin-Cocon L. Diaz O, et al. Front Immunol. 2022 Nov 17;13:1033314. doi: 10.3389/fimmu.2022.1033314. eCollection 2022. Front Immunol. 2022. PMID: 36466918 Free PMC article. Review. - Current and Future Management of Chronic Hepatitis D.
Farci P, Anna Niro G. Farci P, et al. Gastroenterol Hepatol (N Y). 2018 Jun;14(6):342-351. Gastroenterol Hepatol (N Y). 2018. PMID: 30166948 Free PMC article. - Attacking pathogens through their hosts.
Kellam P. Kellam P. Genome Biol. 2006;7(1):201. doi: 10.1186/gb-2006-7-1-201. Epub 2006 Jan 30. Genome Biol. 2006. PMID: 16515720 Free PMC article. Review. - Denying the wolf access to sheep's clothing.
Heller T, Hoofnagle JH. Heller T, et al. J Clin Invest. 2003 Aug;112(3):319-21. doi: 10.1172/JCI19417. J Clin Invest. 2003. PMID: 12897197 Free PMC article. - Recent breakthroughs in the treatment of chronic hepatitis Delta.
Brancaccio G, Gaeta L, Vitale A, Gaeta GB. Brancaccio G, et al. Infez Med. 2022 Jun 1;30(2):204-210. doi: 10.53854/liim-3002-5. eCollection 2022. Infez Med. 2022. PMID: 35693059 Free PMC article. Review.
References
- Rizzetto, M., Ponzetto, A., and Forzani, I. 1991. Epidemiology of hepatitis delta virus: overview. In The hepatitis delta virus. J.L. Gerin, R.H. Purcell, and M. Rizetto, editors. Wiley-Liss Inc. New York, New York, USA. 1–20. - PubMed
- Alter, M.J., and Hadler, S.C. 1993. Delta hepatitis and infection in North America. In Hepatitis delta virus: molecular biology, pathogenesis, and clinical aspects. S.J. Hadziyannis, J.M. Taylor, and F. Bonino, editors. Wiley-Liss Inc. New York, New York, USA. 243–250. - PubMed
- Rizetto, M., and Ponzetto, A. 1995. Hepatitis delta virus infection: medical aspects. In The unique hepatitis delta virus. G. Dinter-Gottlieb, editor. R.C. Landes Publishing Co. Austin, Texas, USA. 125–139.
- Lai MMC. The molecular biology of hepatitis delta virus. Annu. Rev. Biochem. 1995;64:259–286. - PubMed
- Taylor JM. Human hepatitis delta virus: an agent with similarities to certain satellite RNAs of plants. Curr. Top. Microbiol. Immunol. 1999;239:107–122. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials