Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform - PubMed (original) (raw)
Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform
Biserka Mulac-Jericevic et al. Proc Natl Acad Sci U S A. 2003.
Abstract
Progesterone (P) regulates female reproduction via two nuclear receptors, PR-A and PR-B. Although both receptors display overlapping and distinct transcription regulatory properties, their individual physiological roles are unclear. To address the physiological role of PR-A, we generated a mouse model in which expression of PR-B was specifically ablated (PRBKO-/-). We show that selective activation of PR-A in PRBKO-/- mice is sufficient to elicit normal ovarian and uterine responses to P but results in reduced mammary gland morphogenesis. In the absence of PR-B, pregnancy-associated ductal sidebranching and lobuloalveolar development are markedly reduced due to decreased ductal and alveolar epithelial cell proliferation and decreased survival of alveolar epithelium. In an effort to elucidate the molecular genetic signaling pathways that are differentially regulated by PRs in the mammary gland, we have identified receptor activator of nuclear factor kappa B ligand (RANKL) as a paracrine mediator of P-dependent alveologenesis. Further, we demonstrate that the defects in PRBKO-/- mice are associated with an inability of PR-A to activate the RANKL signaling pathway in response to P. Our data indicate that functional interaction between PR-A and PR-B is not required for reproductive activity and that selective modulation of PR-A activity by progestin agonists may have a protective effect against both uterine and mammary gland hyperplasias.
Figures
Fig. 1.
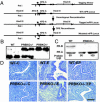
Generation of PRBKO–/– mutant mouse. (A) Strategy for targeted disruption of PR-B expression. Filled boxes, exons; lines, introns; star, mutated ATGB; filled triangles, loxP sites; open box, neo-tk cassette. (B) Southern blot analysis of _Pst_I-digested genomic DNA from WT, heterozygote PRBKO+/–, and homozygote PRBKO–/– mice with cDNA probe encoding nucleotides 20–452 of PR cDNA. (C) Western analysis of uterine extracts to detect PR isoform expression. (D) Hematoxylin/eosin-stained uterine sections from ovariectomized mice treated with oil (C), E, or E+P (EP). (Scale bar, 100 μm.)
Fig. 2.
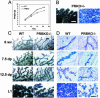
Impaired mammary gland development in mice lacking the PR-B isoform. (A) Neonatal growth curves of offspring from WT and PRBKO–/– mothers (n = 20). Error bars indicate SEM. (B_–_D) Whole-mount (C) and histological (D) analysis by hematoxylin/eosin staining of no. 4 abdominal glands of 6-week-old virgin (6 wv), 7.5 and 12.5 days pregnant (7.5 dp and 12.5 dp), and day 1 lactating (L1) WT and PRBKO–/– mice and PRKO+/– mice at L1 (B). (Scale bars: C, 500 μm; D, 100 μm.)
Fig. 3.
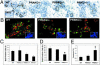
(A) Whole-mount and hematoxylin/eosin analyses of E+P-induced mammary gland morphogenesis in ovariectomized WT, PRBKO–/–, PRAKO–/–, and PRKO+/– mice. (B) Quantitation of tertiary branch points per unit area (seven to nine mice per genotype). (C) Histological staining of WT and PRBKO–/– glands. (D) Immunodetection of alveolar differentiation marker, adipophilin, with rabbit polyclonal IgG followed by Texas red-conjugated anti-rabbit IgG (red). Nuclei were counterstained with DAPI (blue). [Scale bars: A, 500 μm (whole mounts); C, 100 μm (histology); D,20 μm.]
Fig. 4.
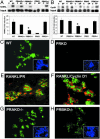
(A) Immunodetection of PRs in WT, PRAKO–/–, PRBKO–/–, and PRKO+/– glands treated with E+P. (Inset) PRKO–/– control. (B) Dual immunofluorescence staining with rabbit polyclonal antibody to PR and mouse monoclonal antibody to BrdUrd. Immunocomplexes were visualized with Texas red (PR, red) and Alexa Fluor 488 (BrdUrd, green). Nuclei were counterstained with DAPI (blue, Insets). (C and D) Cell proliferation in ductal (C) and alveolar (D) epithelium measured by BrdUrd immunohistochemistry. Five thousand cells per mouse were counted, and five mice per genotype were examined. (E) Apoptotic cells in alveolar epithelium determined by TUNEL assay. Ten thousand cells per mouse were counted, and 10 mice per genotype were examined. Statistically significant differences relative to WT are indicated by asterisks (P < 0.05). Error bars (C_–_E) indicate SEM.
Fig. 5.
Decreased activation of RANKL signaling in PRBKO–/– mice. Levels of mRNA transcript for RANKL (A) and cyclin D1 (B) in mammary gland of control and E+P-treated mice were analyzed by RPAs. Values are representative of five independent experiments. Error bars indicate SEM. Statistically significant differences observed when the PRBKO–/– group was compared with WT, PRAKO–/–, or PRKO+/– groups for both RANKL and cyclin D1 (P < 0.05) are indicated by asterisks. (C and_D_) Immunofluorescence staining for RANKL was performed in mammary tissues from E+P-treated WT (C) and PRKO (D) mice. (E and F) Double immunostaining for RANKL and PR (E) and RANKL and cyclin D1 (F) in mammary epithelial cells. Positive cells were visualized with Texas red (PR and cyclin D1, red) and Alexa Fluor 488 (RANKL, green). (G and H) Immunofluorescence staining for RANKL was performed in mammary tissues from E+P-treated PRAKO–/– (G) and PRBKO–/– (H) mice. (C, D, G, and H Insets) Nuclei of the same field counterstained with DAPI.
Fig. 6.
RPA analysis of wnt-4 expression in E+P-treated glands of WT, PRAKO–/–, PRBKO–/–, and PRKO–/– mice relative to L32 control (C). Values are representative of five independent experiments. Error bars indicate SEM.
Similar articles
- Reproductive tissue selective actions of progesterone receptors.
Mulac-Jericevic B, Conneely OM. Mulac-Jericevic B, et al. Reproduction. 2004 Aug;128(2):139-46. doi: 10.1530/rep.1.00189. Reproduction. 2004. PMID: 15280552 Review. - Progesterone-dependent regulation of female reproductive activity by two distinct progesterone receptor isoforms.
Conneely OM, Mulac-Jericevic B, Lydon JP. Conneely OM, et al. Steroids. 2003 Nov;68(10-13):771-8. doi: 10.1016/s0039-128x(03)00126-0. Steroids. 2003. PMID: 14667967 Review. - Reproductive functions of progesterone receptors.
Conneely OM, Mulac-Jericevic B, DeMayo F, Lydon JP, O'Malley BW. Conneely OM, et al. Recent Prog Horm Res. 2002;57:339-55. doi: 10.1210/rp.57.1.339. Recent Prog Horm Res. 2002. PMID: 12017551 Review. - Estrogen-triggered delays in mammary gland gene expression during the estrous cycle: evidence for a novel timing system.
Silberstein GB, Van Horn K, Hrabeta-Robinson E, Compton J. Silberstein GB, et al. J Endocrinol. 2006 Aug;190(2):225-39. doi: 10.1677/joe.1.06725. J Endocrinol. 2006. PMID: 16899557 - Mammary gland development is mediated by both stromal and epithelial progesterone receptors.
Humphreys RC, Lydon J, O'Malley BW, Rosen JM. Humphreys RC, et al. Mol Endocrinol. 1997 Jun;11(6):801-11. doi: 10.1210/mend.11.6.9891. Mol Endocrinol. 1997. PMID: 9171243
Cited by
- Assessment of parenteral estradiol and dihydroxyprogesterone use among other feminizing regimens for transgender women: insights on satisfaction with breast development from community-based healthcare services.
Toffoli Ribeiro C, Gois Í, da Rosa Borges M, Ferreira LGA, Brandão Vasco M, Ferreira JG, Maia TC, Dias-da-Silva MR. Toffoli Ribeiro C, et al. Ann Med. 2024 Dec;56(1):2406458. doi: 10.1080/07853890.2024.2406458. Epub 2024 Sep 20. Ann Med. 2024. PMID: 39301885 Free PMC article. - Structural proteomics defines a sequential priming mechanism for the progesterone receptor.
Mann MD, Wang M, Ferreon JC, Suess MP, Jain A, Malovannaya A, Alvarez RV, Pascal BD, Kumar R, Edwards DP, Griffin PR. Mann MD, et al. bioRxiv [Preprint]. 2024 Oct 3:2024.09.06.611729. doi: 10.1101/2024.09.06.611729. bioRxiv. 2024. PMID: 39282295 Free PMC article. Preprint. - Unraveling the Dynamics of Estrogen and Progesterone Signaling in the Endometrium: An Overview.
Dias Da Silva I, Wuidar V, Zielonka M, Pequeux C. Dias Da Silva I, et al. Cells. 2024 Jul 23;13(15):1236. doi: 10.3390/cells13151236. Cells. 2024. PMID: 39120268 Free PMC article. Review. - IMPACT OF REAL-LIFE ENVIRONMENTAL EXPOSURES ON REPRODUCTION: Evidence for reproductive health effects following exposure to hydraulic fracturing chemical mixtures.
Siegel KR, Bérubé R, Day M, Heldman S, Daley C, Murray BR, Hecht R, Caron-Beaudoin É, Kassotis CD. Siegel KR, et al. Reproduction. 2024 Sep 5;168(4):e240134. doi: 10.1530/REP-24-0134. Print 2024 Oct 1. Reproduction. 2024. PMID: 39074054 Review. - C/EBPβ deletion in macrophages impairs mammary gland alveolar budding during the estrous cycle.
Rojo MD, Bandyopadhyay I, Burke CM, Sturtz AD, Phillips ES, Matherne MG, Embrey SJ, LaRue R, Qiu Y, Schwertfeger KL, Machado HL. Rojo MD, et al. Life Sci Alliance. 2024 Jul 18;7(10):e202302516. doi: 10.26508/lsa.202302516. Print 2024 Oct. Life Sci Alliance. 2024. PMID: 39025525 Free PMC article.
References
- Conneely, O. M., Mulac-Jericevic, B., DeMayo, F., Lydon, J. P. & O'Malley, B. W. (2002) Recent Prog. Horm. Res. 57, 339–355. - PubMed
- Lydon, J. P., DeMayo, F. J., Funk, C. R., Mani, S. K., Hughes, A. R., Montgomery, C. A., Jr., Shyamala, G., Conneely, O. M. & O'Malley, B. W. (1995) Genes Dev. 9, 2266–2278. - PubMed
- Conneely, O. M., Kettelberger, D. M., Tsai, M.-J., Schrader, W. T. & O'Malley, B. W. (1989) J. Biol. Chem. 264, 14062–14064. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials