Hrs regulates multivesicular body formation via ESCRT recruitment to endosomes - PubMed (original) (raw)
Hrs regulates multivesicular body formation via ESCRT recruitment to endosomes
Kristi G Bache et al. J Cell Biol. 2003.
Abstract
Hrs and the endosomal sorting complexes required for transport, ESCRT-I, -II, and -III, are involved in the endosomal sorting of membrane proteins into multivesicular bodies and lysosomes or vacuoles. The ESCRT complexes are also required for formation of intraluminal endosomal vesicles and for budding of certain enveloped RNA viruses such as HIV. Here, we show that Hrs binds to the ESCRT-I subunit Tsg101 via a PSAP motif that is conserved in Tsg101-binding viral proteins. Depletion of Hrs causes a reduction in membrane-associated ESCRT-I subunits, a decreased number of multivesicular bodies and an increased size of late endosomes. Even though Hrs mainly localizes to early endosomes and Tsg101 to late endosomes, the two proteins colocalize on a subpopulation of endosomes that contain lyso-bisphosphatidic acid. Overexpression of Hrs causes accumulation of Tsg101 on early endosomes and prevents its localization to late endosomes. We conclude that Hrs mediates the initial recruitment of ESCRT-I to endosomes and, thereby, indirectly regulates multivesicular body formation.
Figures
Figure 1.
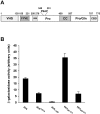
Hrs interacts with Tsg101. (A) A schematic representation of Hrs. The VHS, FYVE, coiled-coil (CC), and clathrin-binding domain (CBD) are indicated. The ubiquitin interacting motif (UIM), PSAP sequence, and proline- and proline/glutamine-rich regions are also indicated. (B) Interaction between Hrs and Tsg101 in the yeast two-hybrid system. The indicated Hrs constructs were used as bait and Tsg101 as prey. The values indicate β-galactosidase activities in arbitrary units. Neither of the bait constructs showed any significant reporter activation (<1 U) in the absence of a prey construct. All determinations were done in triplicate. Error bars denote SEM. (C) Interaction between Hrs and Tsg101 in vitro. GST alone or fused to Hrs287–573 or Hrs287–573 P351A were immobilized on glutathione-Sepharose beads and incubated with in vitro–translated 35S-labeled Tsg101 for 1 h at 4°C. The beads were washed and analyzed by SDS-PAGE and fluorography. The amounts bound in lanes 1 and 7 correspond to 2–3% of the input amount. The doublet band is presumably due to translational initiation downstream of the initiator ATG.
Figure 1.
Hrs interacts with Tsg101. (A) A schematic representation of Hrs. The VHS, FYVE, coiled-coil (CC), and clathrin-binding domain (CBD) are indicated. The ubiquitin interacting motif (UIM), PSAP sequence, and proline- and proline/glutamine-rich regions are also indicated. (B) Interaction between Hrs and Tsg101 in the yeast two-hybrid system. The indicated Hrs constructs were used as bait and Tsg101 as prey. The values indicate β-galactosidase activities in arbitrary units. Neither of the bait constructs showed any significant reporter activation (<1 U) in the absence of a prey construct. All determinations were done in triplicate. Error bars denote SEM. (C) Interaction between Hrs and Tsg101 in vitro. GST alone or fused to Hrs287–573 or Hrs287–573 P351A were immobilized on glutathione-Sepharose beads and incubated with in vitro–translated 35S-labeled Tsg101 for 1 h at 4°C. The beads were washed and analyzed by SDS-PAGE and fluorography. The amounts bound in lanes 1 and 7 correspond to 2–3% of the input amount. The doublet band is presumably due to translational initiation downstream of the initiator ATG.
Figure 2.
Hrs is required for the efficient association of Tsg101 with membranes. HeLa cells treated with control RNA (−) or with siRNA against Hrs (+) were fractionated into membrane and cytosolic fractions as described in Materials and methods. Hrs left in the cytosol and on membranes after siRNA treatment, as described in Materials and methods, was analyzed by SDS-PAGE (A and B, top lanes), and the corresponding levels of Tsg101 and hVps28 were shown by sequential blotting of the same membrane with anti-Tsg101 (A) or anti-hVps28 (B). The loaded amount of membrane fraction was sixfold higher than that of the cytosol fraction in A, and twofold higher in B. To visualize transferred proteins, the blots were stained with Ponceau S (bottom panels) before detection of Hrs and Tsg101 (A), and Hrs and hVps28 (B). The relative intensities of the bands from membrane fractions of control and siRNA-treated cells were quantified using ImageQuant 5.0 (C), and are presented as the average of three experiments. Error bars denote SEM.
Figure 3.
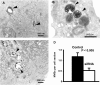
Hrs is required for MVB formation. HeLa cells treated with control RNA or siRNA against Hrs were incubated with 5 mg/ml HRP for 15 min and processed for electron microscopy. In control cells, we observed early endosomes of varying sizes (A) and MVBs (B). (C) In siRNA-treated cells we also observed early endosomes, but significantly less MVBs. (A–C) Arrowheads indicate HRP-positive structures. (D) To quantify the effect of siRNA on MVB formation, we estimated the number of MVBs per cell section. We included only MVBs with an appearance as seen in B in the estimation and omitted early endosomal structures as seen in A and C. MVBs were counted and expressed as the mean number of MVBs per cell section. Three separate experiments were performed and 20 cells were counted for each condition. Statistical significance was estimated with the t test. Error bars denote SEM.
Figure 4.
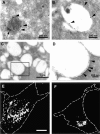
Hrs depletion affects the morphology of late endosomes and lysosomes. HeLa cells treated with control double-stranded RNA (A and B) or siRNA against Hrs (C and D) were prepared for electron microscopy or immunofluorescence microscopy as described in Materials and methods. Late endosomes and lysosomes were visualized by 7 nm internalized BSA gold (A, arrow) or staining with antibodies against LAMP-2 followed by 15 nm protein A–gold (A–D, arrowheads). The boxed area in C is shown magnified in D. Note the different sizes of bars. Immunofluorescence images were obtained by labeling with antibodies against LAMP-1 (E and F). Bar, (E and F) 5 μm. E shows a control cell, whereas F shows an siRNA-treated cell.
Figure 5.
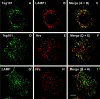
Tsg101 has a broader endosomal distribution than Hrs. HeLa cells grown on coverslips were permeabilized before fixation and double labeled with anti-Tsg101 and anti–LAMP-1 (A–C), anti-Tsg101 and Hrs (D–F), or anti-Hrs and anti–LAMP-1 (G–I). Yellow color in the merged images (C, F, and I) indicates colocalization. Bar, 5 μm.
Figure 6.
Tsg101 and Hrs colocalize on LBPA-containing endosomes. HeLa cells were permeabilized before fixation and labeled with anti-Hrs (C) and anti-LBPA (B) primary and secondary antibodies before staining with Zenon®-labeled anti-Tsg101 (A) as described in Materials and methods. Colocalization between LBPA and Tsg101 is shown in yellow (D), between LBPA and Hrs in turquoise (E), and between all three molecules in white (F). Examples of profiles positive for all three molecules are indicated by arrows. Bar, 5 μm.
Figure 7.
Overexpression of Hrs prevents the localization of Tsg101 to late endosomes. HeLa cells were transfected (D–L) or not (A–C) with Hrs for 48 h and permeabilized before fixation. The cells were labeled with anti-Tsg101 (A, D, and G), anti-EEA1 (B and H), or anti-LAMP-1 (I). Yellow (C, F, and J) or purple (K) color in merged images indicates colocalization. An interference contrast image of the Hrs-transfected cell (G–K) is shown in L (colors are inverted in this panel). Bar, 5 μm.
Similar articles
- Ultrastructural analysis of ESCRT proteins suggests a role for endosome-associated tubular-vesicular membranes in ESCRT function.
Welsch S, Habermann A, Jäger S, Müller B, Krijnse-Locker J, Kräusslich HG. Welsch S, et al. Traffic. 2006 Nov;7(11):1551-66. doi: 10.1111/j.1600-0854.2006.00489.x. Epub 2006 Oct 2. Traffic. 2006. PMID: 17014699 - TSG101 interaction with HRS mediates endosomal trafficking and receptor down-regulation.
Lu Q, Hope LW, Brasch M, Reinhard C, Cohen SN. Lu Q, et al. Proc Natl Acad Sci U S A. 2003 Jun 24;100(13):7626-31. doi: 10.1073/pnas.0932599100. Epub 2003 Jun 11. Proc Natl Acad Sci U S A. 2003. PMID: 12802020 Free PMC article. - HIV Gag mimics the Tsg101-recruiting activity of the human Hrs protein.
Pornillos O, Higginson DS, Stray KM, Fisher RD, Garrus JE, Payne M, He GP, Wang HE, Morham SG, Sundquist WI. Pornillos O, et al. J Cell Biol. 2003 Aug 4;162(3):425-34. doi: 10.1083/jcb.200302138. J Cell Biol. 2003. PMID: 12900394 Free PMC article. - A protein's final ESCRT.
Babst M. Babst M. Traffic. 2005 Jan;6(1):2-9. doi: 10.1111/j.1600-0854.2004.00246.x. Traffic. 2005. PMID: 15569240 Review. - Endosomal and non-endosomal functions of ESCRT proteins.
Slagsvold T, Pattni K, Malerød L, Stenmark H. Slagsvold T, et al. Trends Cell Biol. 2006 Jun;16(6):317-26. doi: 10.1016/j.tcb.2006.04.004. Epub 2006 May 22. Trends Cell Biol. 2006. PMID: 16716591 Review.
Cited by
- Late domain dependent E-cadherin recruitment into extracellular vesicles.
Bänfer S, Kutscher S, Fleck F, Dienst M, Preußer C, Pogge von Strandmann E, Jacob R. Bänfer S, et al. Front Cell Dev Biol. 2022 Sep 7;10:878620. doi: 10.3389/fcell.2022.878620. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36172289 Free PMC article. - Vps34 regulates Rab7 and late endocytic trafficking through recruitment of the GTPase-activating protein Armus.
Jaber N, Mohd-Naim N, Wang Z, DeLeon JL, Kim S, Zhong H, Sheshadri N, Dou Z, Edinger AL, Du G, Braga VM, Zong WX. Jaber N, et al. J Cell Sci. 2016 Dec 1;129(23):4424-4435. doi: 10.1242/jcs.192260. Epub 2016 Oct 28. J Cell Sci. 2016. PMID: 27793976 Free PMC article. - The role of WWP1-Gag interaction and Gag ubiquitination in assembly and release of human T-cell leukemia virus type 1.
Heidecker G, Lloyd PA, Soheilian F, Nagashima K, Derse D. Heidecker G, et al. J Virol. 2007 Sep;81(18):9769-77. doi: 10.1128/JVI.00642-07. Epub 2007 Jul 3. J Virol. 2007. PMID: 17609263 Free PMC article. - Structural and functional organization of the ESCRT-I trafficking complex.
Kostelansky MS, Sun J, Lee S, Kim J, Ghirlando R, Hierro A, Emr SD, Hurley JH. Kostelansky MS, et al. Cell. 2006 Apr 7;125(1):113-26. doi: 10.1016/j.cell.2006.01.049. Cell. 2006. PMID: 16615894 Free PMC article. - A genome-wide screen for Saccharomyces cerevisiae deletion mutants that affect telomere length.
Askree SH, Yehuda T, Smolikov S, Gurevich R, Hawk J, Coker C, Krauskopf A, Kupiec M, McEachern MJ. Askree SH, et al. Proc Natl Acad Sci U S A. 2004 Jun 8;101(23):8658-63. doi: 10.1073/pnas.0401263101. Epub 2004 May 25. Proc Natl Acad Sci U S A. 2004. PMID: 15161972 Free PMC article.
References
- Babst, M., G. Odorizzi, E.J. Estepa, and S.D. Emr. 2000. Mammalian tumor susceptibility gene 101 (TSG101) and the yeast homologue, Vps23p, both function in late endosomal trafficking. Traffic. 1:248–258. - PubMed
- Babst, M., D.J. Katzmann, E.J. Estepa-Sabal, T. Merloo, and S.D. Emr. 2002. a. ESCRT-III: an endosome-associated heterooligomeric protein complex required for MVB sorting. Dev. Cell. 3:271–282. - PubMed
- Babst, M., D.J. Katzmann, W.B. Snyder, B. Wendland, and S.D. Emr. 2002. b. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular bodies. Dev. Cell. 3:283–289. - PubMed
- Bache, K.G., C. Raiborg, A. Mehlum, and H. Stenmark. 2003. STAM and Hrs are subunits of a multivalent ubiquitin-binding complex on early endosomes. J. Biol. Chem. 278:12513–12521. - PubMed
- Bilodeau, P.S., J.L. Urbanowski, S.C. Winistorfer, and R.C. Piper. 2002. The Vps27p Hse1p complex binds ubiquitin and mediates endosomal protein sorting. Nat. Cell Biol. 4:534–539. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous