Genetic disorders of the skeleton: a developmental approach - PubMed (original) (raw)
Review
Genetic disorders of the skeleton: a developmental approach
Uwe Kornak et al. Am J Hum Genet. 2003 Sep.
Abstract
Although disorders of the skeleton are individually rare, they are of clinical relevance because of their overall frequency. Many attempts have been made in the past to identify disease groups in order to facilitate diagnosis and to draw conclusions about possible underlying pathomechanisms. Traditionally, skeletal disorders have been subdivided into dysostoses, defined as malformations of individual bones or groups of bones, and osteochondrodysplasias, defined as developmental disorders of chondro-osseous tissue. In light of the recent advances in molecular genetics, however, many phenotypically similar skeletal diseases comprising the classical categories turned out not to be based on defects in common genes or physiological pathways. In this article, we present a classification based on a combination of molecular pathology and embryology, taking into account the importance of development for the understanding of bone diseases.
Figures
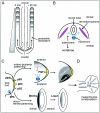
Figure 1
Patterning of the axial and appendicular skeleton. A, Somitogenesis. Presomitic mesoderm on both sides of the neural tube becomes subdivided into somites in craniocaudal direction. To illustrate the spatial and temporal coordination of somite formation, a time difference of 90 min between the left and right portion of the presomitic mesoderm is depicted here. Waves of notch expression originating from the caudal pole of the embryo time the somite border formation so that a new somite is formed every 90 min. Spacial coordination is conveyed by a gradient of FGF8 expression. Gray shading of newly formed somites represents polarized expression of Dll3. Somites give rise to several structures, among them the axial skeleton. B, Dorsoventral patterning of the axial skeleton. Each somite subdivides into a dermatomyotome, from which the muscles of the extremities are derived, and a sclerotome, which develops into the axial skeleton. This process is regulated by Shh, which is expressed by the notochord. C, Patterning of the limb buds. The AER is an important signaling center for proximodistal outgrowth of limb structures. p63 expression is crucial for sustaining the AER. The ZPA directs anteroposterior patterning by expression of Shh. Under the influence of Shh, the repressor form of Gli3, Gli3R, is converted to the activating form Gli3A, and a gradient of Shh and Gli3 expression is established. Expression of the Hoxd-cluster genes occurs in a highly ordered fashion as a result of the Shh/Gli3 gradient created by the ZPA. Wnt7a shows a polarized expression, which is strongest in the dorsal ectoderm of the limb bud. It induces Lmx1 in the underlying mesenchyme, which has a key role in dorsoventral patterning. D, The result of the patterning process becomes visible as mesenchymal condensations at later developmental stages. These condensations either develop into cartilaginous bone precursors in endochondral ossification or are directly transformed into bone in desmal ossification.
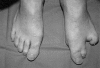
Figure 2
SHFM caused by mutations in p63. Whereas the middle toes of the left foot show the typical features of the disorder, the right foot appears to be only mildly affected, illustrating the very variable and often asymmetric manifestation. The phenotype is thought to arise from an insufficiency of the AER during patterning of the distal limb structures.
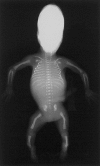
Figure 3
Campomelic dysplasia in a stillborn fetus. Note the hallmarks of the disorder: bowing of femora and tibiae, hypoplasia of scapulae and pelvis, and a small, bell-shaped thorax with a decreased number of ribs. SOX9 mutations cause a defective formation of early condensations that precede the formation of a cartilaginous skeleton.
Figure 4
Chondrocyte differentiation and proliferation in the growth plate. Chondrocytes are arranged in four layers, representing their stages of differentiation: (i) resting, (ii) proliferating, (iii) prehypertrophic, and (iv) hypertrophic/terminal hypertrophic chondrocytes. The latter are replaced by bone by the joint action of osteoblasts (green) and multinuclear osteoclasts (blue). Genes that are characteristically expressed within each layer are listed on the right side. A complex signaling network regulates proliferation and differentiation of chondrocytes. Prehypertrophic chondrocytes express Ihh, which regulates PTHrP, which is expressed in the joint region, via the perichondrium. PTHrP, in turn, inhibits differentiation of proliferating chondrocytes. FGFs inhibit chondrocyte proliferation and stimulate their differentiation. BMPs act antagonistically to the FGFs. The intensity of gray shading indicates the degree of calcification of the extracellular matrix.
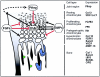
Figure 5
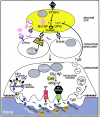
Function and regulation of the osteoclast. Osteoblasts/stromal cells (yellow) regulate proliferation, differentiation, and activity of the osteoclast via RANKL and M-CSF. M-CSF binds to its receptor, c-fms, on mononuclear osteoclast precursors and stimulates their proliferation. RANKL stimulates differentiation into multinuclear resorbing osteoclasts via RANK, which transduces the signal with the help of TRAF6 and NFκB. As soon as the mature osteoclast attaches to the bone surface, it forms a ruffled border membrane containing large amounts of H+-ATPase complexes (green) and the chloride channel ClC-7 (red), which together transport HCl and acidify the resorption lacuna. Protons are provided by the cytosolic carbonic anhydrase type II (CAII). Acidic proteases like cathepsin k (blue circles) become active and degrade the bone matrix proteins (mainly collagen) and release bound growth factors, above all Tgfβ. Tgfβ is able to signal back to the osteoblast establishing a negative feedback loop by stimulating OPG expression. Degradation products are transported out of the cell by transcytosis. K= cathepsin k.
Figure 6
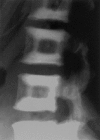
Sandwich vertebra in ADOII. ADOII can be diagnosed by characteristic radiological findings. The radiograph shows the typical sandwichlike appearance of the vertebra by thickening of the endplates.
Similar articles
- Molecular-pathogenetic classification of genetic disorders of the skeleton.
Superti-Furga A, Bonafé L, Rimoin DL. Superti-Furga A, et al. Am J Med Genet. 2001 Winter;106(4):282-93. Am J Med Genet. 2001. PMID: 11891680 Review. - International nosology and classification of constitutional disorders of bone (2001).
Hall CM. Hall CM. Am J Med Genet. 2002 Nov 15;113(1):65-77. doi: 10.1002/ajmg.10828. Am J Med Genet. 2002. PMID: 12400068 - Radiological diagnosis of the constitutional disorders of bone. As easy as A, B, C?
Offiah AC, Hall CM. Offiah AC, et al. Pediatr Radiol. 2003 Mar;33(3):153-61. doi: 10.1007/s00247-002-0855-8. Epub 2002 Dec 20. Pediatr Radiol. 2003. PMID: 12612812 Review. - [Genetic basis for skeletal disease. Radiological approach for genetic skeletal disorders].
Nishimura G. Nishimura G. Clin Calcium. 2010 Aug;20(8):1175-81. Clin Calcium. 2010. PMID: 20675927 Review. Japanese. - Genetic analysis of skeletal dysplasia: recent advances and perspectives in the post-genome-sequence era.
Ikegawa S. Ikegawa S. J Hum Genet. 2006;51(7):581-6. doi: 10.1007/s10038-006-0401-x. Epub 2006 May 3. J Hum Genet. 2006. PMID: 16670815 Review.
Cited by
- Polydactyly: Clinical and molecular manifestations.
Kyriazis Z, Kollia P, Grivea I, Stefanou N, Sotiriou S, Dailiana ZH. Kyriazis Z, et al. World J Orthop. 2023 Jan 18;14(1):13-22. doi: 10.5312/wjo.v14.i1.13. eCollection 2023 Jan 18. World J Orthop. 2023. PMID: 36686282 Free PMC article. Review. - Homeobox genes d11-d13 and a13 control mouse autopod cortical bone and joint formation.
Villavicencio-Lorini P, Kuss P, Friedrich J, Haupt J, Farooq M, Türkmen S, Duboule D, Hecht J, Mundlos S. Villavicencio-Lorini P, et al. J Clin Invest. 2010 Jun;120(6):1994-2004. doi: 10.1172/JCI41554. Epub 2010 May 10. J Clin Invest. 2010. PMID: 20458143 Free PMC article. - Case report: multiple fractures in a patient with mutations of TWIST1 and TNSALP.
Barvencik F, Gebauer M, Schinke T, Amling M. Barvencik F, et al. Clin Orthop Relat Res. 2008 Apr;466(4):990-6. doi: 10.1007/s11999-008-0123-9. Epub 2008 Jan 25. Clin Orthop Relat Res. 2008. PMID: 18219546 Free PMC article. - GMNN and DLL1 mutation-related spondylocarpotarsal synostosis: a case report.
Lee J, Ryu B, Kim Y, Lee E. Lee J, et al. J Yeungnam Med Sci. 2025;42:15. doi: 10.12701/jyms.2024.01137. Epub 2024 Dec 11. J Yeungnam Med Sci. 2025. PMID: 39659197 Free PMC article. - High bone mass in the STR/ort mouse results from increased bone formation and impaired bone resorption and is associated with extramedullary hematopoiesis.
Pasold J, Engelmann R, Keller J, Joost S, Marshall RP, Frerich B, Müller-Hilke B. Pasold J, et al. J Bone Miner Metab. 2013 Jan;31(1):71-81. doi: 10.1007/s00774-012-0394-9. Epub 2012 Nov 29. J Bone Miner Metab. 2013. PMID: 23192248
References
Electronic-Database Information
- Health Supervision for Children with Achondroplasia, American Academy of Pediatrics, http://www.aap.org/policy/00696.html - PubMed
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for postaxial polydactyly type I, preaxial polydactyly type IV, Greig cephalopolysyndactyly, dominant Pallister-Hall syndrome, TBS, TPTPS, EEC, limb-mammary syndrome, nonsyndromic SHFM, Hay-Wells syndrome, SHFM1, HOS, Okihiro syndrome, ulnar-mammary syndrome, acheiropodia, NPS, SPD, hand-foot-genital syndrome, amegakaryocytic thrombocytopenia and radioulnar synostosis, BDA1, BDB, BDC, chondrodysplasia [Grebe type], chondrodysplasia [Hunter-Thompson type], Du Pan syndrome, proximal symphalangism, multiple-synostosis syndrome, campomelic dysplasia, Ellis–van Creveld syndrome, CCD, LWD, Langer mesomelic dysplasia, multiple cartilaginous exostoses, tricho-rhino-phalangeal syndrome, Blomstrand chondrodysplasia, Jansen type metaphyseal chondrodysplasia, enchondromatosis, achondroplasia, hypochondroplasia, thanatophoric dysplasia, autosomal dominant hypophosphatemic rickets, recessive Robinow syndrome, achondrogenesis type II, hypochondrogenesis, SEDc, Kniest dysplasia, Stickler dysplasia, otospondylomegaepiphyseal dysplasia, EDM2, EDM3, EDM1, pseudoachondroplasia, EDM5, EDM4, diastrophic dysplasia, atelosteogenesis type 2, achondrogenesis type 1B, dyssegmental dysplasia [Silverman-Handmaker type], Schwartz-Jampel syndrome, hypophosphatasia, CMD, OPS, HBM, ADOI, sclerosteosis, van Buchem disease, juvenile Paget disease, familial expansile osteolysis, Camurati-Engelmann syndrome, idiopathic osteolysis, ARO, osteopetrosis with renal tubular acidosis, ADOII, and pycnodysostosis)
References
- ADHR Consortium, The (2000) Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet 26:345–348 - PubMed
- Afzal AR, Rajab A, Fenske CD, Oldridge M, Elanko N, Ternes-Pereira E, Tuysuz B, Murday VA, Patton MA, Wilkie AO, Jeffery S (2000) Recessive Robinow syndrome, allelic to dominant brachydactyly type B, is caused by mutation of ROR2. Nat Genet 25:419–422 - PubMed
- Albrecht AN, Schwabe GC, Stricker S, Boddrich A, Wanker EE, Mundlos S (2002) The synpolydactyly homolog (spdh) mutation in the mouse—a defect in patterning and growth of limb cartilage elements. Mech Dev 112:53–67 - PubMed
- Arikawa-Hirasawa E, Watanabe H, Takami H, Hassell JR, Yamada Y (1999) Perlecan is essential for cartilage and cephalic development. Nat Genet 23:354–358 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous