Nuclear import of hepatitis B virus capsids and release of the viral genome - PubMed (original) (raw)
Nuclear import of hepatitis B virus capsids and release of the viral genome
Birgit Rabe et al. Proc Natl Acad Sci U S A. 2003.
Abstract
While studying the import of the hepatitis B virus genome into the nucleus of permeabilized tissue culture cells, we found that viral capsids were imported in intact form through the nuclear pore into the nuclear basket. Import depended on phosphorylation of the capsid protein and was mediated by the cellular transport receptors importin alpha and beta. Virus-derived capsids that contained the mature viral genome were able to release the viral DNA and capsid protein into the nucleoplasm. The uncoating reaction was independent of Ran, a GTP-binding enzyme responsible for dissociating other imported cargo from the inner face of the nuclear pore. Immature capsids that did not contain the mature viral genome reached the basket but did not release capsid proteins nor immature genomes into the nucleoplasm. The different fate of mature and immature capsids after passing the nuclear pore indicates that the outcome of a nuclear import event may be regulated within the nuclear basket.
Figures
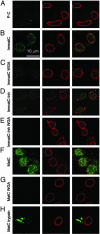
Fig. 1.
Localization of hepatitis B capsids in digitonin-permeabilized HuH-7 cells by confocal laser scanning microscopy after immune staining. Capsids (green,Left) and NPCs (red, Center) were stained by indirect immunofluorescence. (Right) Merged images are shown. In all samples, ATP and an ATP-generating system were present, and rabbit reticulocyte lysate was used as the source of cytosolic proteins. (A) P-C do not bind to cellular structures. (B) Nuclear binding of ImmatC. (C) ImmatC binding is inhibited when WGA is added. (D) ImmatC-Inh shows a weaker nuclear binding than ImmatC. (E) Like that of ImmatC, this binding is inhibited when WGA is added. (F) MatC generated intranuclear capsids. (G) The formation of intranuclear capsids is inhibited by WGA. (H) Tryptic digestion of MatC prevents nuclear binding and import. All pictures were taken at the same magnification.
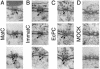
Fig. 2.
Intact HBV capsids are able to cross the nuclear pores. Views of nuclear envelope cross sections with adjacent cytoplasm (c) and nucleoplasm (n) from a_Xenopus_ oocyte that has been microinjected with MatC (A), ImmatC (B), and EcPC (C). Arrowheads point to capsids associated with the nuclear face of the NPC. (D) Nuclear envelope cross section from a control (noninjected) Xenopus oocyte. (Scale bars = 100 nm.) Histograms showing the size distribution of the capsids associated with the cytoplasmic or the nuclear face of the nuclear pore are given in Fig. 7.
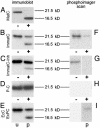
Fig. 3.
Maturation-dependent structural changes of capsids. +, Capsids digested with immobilized trypsin after phosphorylation with 32P. The capsid proteins were visualized by immunoblotting (A_–_E), using an antibody against denatured capsid protein, or by phosphorimaging (F_–_I) after SDS/PAGE. Nondigested controls (–) were visualized in the same way. (A) In MatC, all capsid proteins were cleaved to 16.5 kDa. (B) The digestion of ImmatC resulted predominantly in capsid proteins of the size of undegraded protein (21.5 kDa), but the same degradation products of 16.5 kDa. (C) When ImmatC-Inh were digested, the same pattern was observable, but the amount of degradation product was reduced. (D and E) The capsid protein of the P-C was not degraded (D) as the unphosphorylated EcC (E, lane u). In contrast, tryptic digestion of EcPC generated the same degradation products as ImmatC (E, lane p).
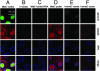
Fig. 4.
Release of HBV genomes from the capsids in the presence of cytosolic proteins. Released HBV genomes were detected by native fluorescence in situ hybridization (FITC-labeled probe; green); capsids (in red) and NPCs (in blue) were detected by immunostaining. The merges are shown in the bottom row. (A) MatC were subjected to transport conditions, and hybridization was performed by using a probe against the single-stranded region of the mature HBV genome. (B) No capsids were added. (C) Inhibition of the nuclear transport of MatC by WGA using the same probe as in A.(D) Nuclear transport was as in A, but the hybridization was performed with the counterstrand probe. (E and F) ImmatC were added to the import reaction. (E) A weak hybridization signal was observed by using the probe against the single-stranded region of the mature HBV genome. (F) No signal was observed when the hybridization was performed with the counterstrand probe, indicating that capsids containing the RNA precursor do not release the genome.
Fig. 5.
The release of the HBV genome is Ran-independent. (Top) Localization of the released viral genomes (in green) from MatC. (Middle) Capsids in the same cells (in red). (Bottom) Corresponding nuclear import of a simian virus 40 T antigen (SV40TAg) NLS-FITC-labeled BSA conjugate (in green). (Top) The costain against the NPCs is depicted in blue. (Bottom) This control stain is shown in red. (A) Impα, Impβ, and Ran replaced the cytosolic proteins. Intranuclear genomes and capsids were generated, and the control conjugate was imported. (B) Positive control, in which rabbit reticulocyte lysate (RRL) was added. As in A, released intranuclear genomes and capsids could be observed. (C and D) Control reactions in which no transport factors were present (C) or in which no substrates were added (D). (E) Cytosolic proteins were replaced by Impα and Impβ, resulting in the generation of intranuclear genomes and capsids, but not in a nuclear import of the control conjugate. (F) Impα, Impβ, Ran, and GTP[γS] were added. Intranuclear genomes and capsids were generated to the same extent as in E, but the nuclear import of the control conjugate was strongly reduced.
Similar articles
- Nucleoporin 153 arrests the nuclear import of hepatitis B virus capsids in the nuclear basket.
Schmitz A, Schwarz A, Foss M, Zhou L, Rabe B, Hoellenriegel J, Stoeber M, Panté N, Kann M. Schmitz A, et al. PLoS Pathog. 2010 Jan 29;6(1):e1000741. doi: 10.1371/journal.ppat.1000741. PLoS Pathog. 2010. PMID: 20126445 Free PMC article. - Cytoplasmic Parvovirus Capsids Recruit Importin Beta for Nuclear Delivery.
Mäntylä E, Aho V, Kann M, Vihinen-Ranta M. Mäntylä E, et al. J Virol. 2020 Jan 31;94(4):e01532-19. doi: 10.1128/JVI.01532-19. Print 2020 Jan 31. J Virol. 2020. PMID: 31748386 Free PMC article. - Nuclear entry of hepatitis B virus capsids involves disintegration to protein dimers followed by nuclear reassociation to capsids.
Rabe B, Delaleau M, Bischof A, Foss M, Sominskaya I, Pumpens P, Cazenave C, Castroviejo M, Kann M. Rabe B, et al. PLoS Pathog. 2009 Aug;5(8):e1000563. doi: 10.1371/journal.ppat.1000563. Epub 2009 Aug 28. PLoS Pathog. 2009. PMID: 19714236 Free PMC article. - Nuclear Import of Hepatitis B Virus Capsids and Genome.
Gallucci L, Kann M. Gallucci L, et al. Viruses. 2017 Jan 21;9(1):21. doi: 10.3390/v9010021. Viruses. 2017. PMID: 28117723 Free PMC article. Review. - Protoparvovirus Knocking at the Nuclear Door.
Mäntylä E, Kann M, Vihinen-Ranta M. Mäntylä E, et al. Viruses. 2017 Oct 2;9(10):286. doi: 10.3390/v9100286. Viruses. 2017. PMID: 28974036 Free PMC article. Review.
Cited by
- Formation of hepatitis B virus covalently closed circular DNA: removal of genome-linked protein.
Gao W, Hu J. Gao W, et al. J Virol. 2007 Jun;81(12):6164-74. doi: 10.1128/JVI.02721-06. Epub 2007 Apr 4. J Virol. 2007. PMID: 17409153 Free PMC article. - Identification and characterization of a novel bipartite nuclear localization signal in the hepatitis B virus polymerase.
Lupberger J, Schaedler S, Peiran A, Hildt E. Lupberger J, et al. World J Gastroenterol. 2013 Nov 28;19(44):8000-10. doi: 10.3748/wjg.v19.i44.8000. World J Gastroenterol. 2013. PMID: 24307793 Free PMC article. - The roles of the nuclear pore complex in cellular dysfunction, aging and disease.
Sakuma S, D'Angelo MA. Sakuma S, et al. Semin Cell Dev Biol. 2017 Aug;68:72-84. doi: 10.1016/j.semcdb.2017.05.006. Epub 2017 May 12. Semin Cell Dev Biol. 2017. PMID: 28506892 Free PMC article. Review. - The Structural Biology of Hepatitis B Virus: Form and Function.
Venkatakrishnan B, Zlotnick A. Venkatakrishnan B, et al. Annu Rev Virol. 2016 Sep 29;3(1):429-451. doi: 10.1146/annurev-virology-110615-042238. Epub 2016 Aug 1. Annu Rev Virol. 2016. PMID: 27482896 Free PMC article. Review. - Hepatitis B Virus Covalently Closed Circular DNA Formation in Immortalized Mouse Hepatocytes Associated with Nucleocapsid Destabilization.
Cui X, Guo JT, Hu J. Cui X, et al. J Virol. 2015 Sep;89(17):9021-8. doi: 10.1128/JVI.01261-15. Epub 2015 Jun 17. J Virol. 2015. PMID: 26085156 Free PMC article.
References
- Whittaker, G. R., Kann, M. & Helenius, A. (2000) Annu. Rev. Cell Dev. Biol. 16, 627–657. - PubMed
- Kann, M., Thomssen, R., Kochel, H. G. & Gerlich, W. H. (1993) Arch. Virol. 8, Suppl., 53–62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous