Activation of phospholipase D by the small GTPase Sar1p is required to support COPII assembly and ER export - PubMed (original) (raw)
Activation of phospholipase D by the small GTPase Sar1p is required to support COPII assembly and ER export
Purnima Pathre et al. EMBO J. 2003.
Abstract
The small GTPase Sar1p controls the assembly of the cytosolic COPII coat that mediates export from the endoplasmic reticulum (ER). Here we demonstrate that phospholipase D (PLD) activation is required to support COPII-mediated ER export. PLD activity by itself does not lead to the recruitment of COPII to the membranes or ER export. However, PLD activity is required to support Sar1p-dependent membrane tubulation, the subsequent Sar1p-dependent recruitment of Sec23/24 and Sec13/31 COPII complexes to ER export sites and ER export. Sar1p recruitment to the membrane is PLD independent, yet activation of Sar1p is required to stimulate PLD activity on ER membranes, thus PLD is temporally regulated to support ER export. Regulated modification of membrane lipid composition is required to support the cooperative interactions that enable selective transport, as we demonstrate here for the mammalian COPII coat.
Figures
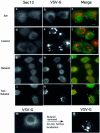
Fig. 1. Phospholipase D is required to support ER export in vivo. Infected NRK cells expressing TsO45 VSV-G were incubated on ice (a–c) or at 32°C (d–n) in the absence (d–f) or presence (g–i) of 1-butanol (1.5%) or tert-butanol (1.5%) (j–l) for 20 min. At the end of incubations, the cells were transferred to ice, permeabilized, fixed and the localization of Sec13 (a, d, g and j) and VSV-G (b, e, h, k, m and n) was determined using indirect immunofluorescence. Export of VSV-G from the ER and delivery to the Golgi (compare h with e and k) and Sec13 binding to the membranes (compare a, d and j with g) were inhibited by 1-butanol but not by tert-butanol (the arrows in c, f and l indicate areas of punctate Sec13 staining). In (m), cells were incubated for 20 min in the presence of 1-butanol. At 20 min, the medium was replaced with fresh medium without butanol and the cells were incubated further for 20 min before fixation and analysis (n). Following butanol removal, VSV-G was delivered to the Golgi complex efficiently. The bar in (c) is 5 µm. A representative experiment is shown; similar results were obtained in three separate experiments.
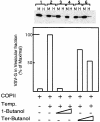
Fig. 2. PLD activity is required for COPII-mediated export from the ER. VSV-G-containing membranes were incubated with purified COPII components [Sar1p-H79G (2 µg) Sec23/24 (1 µg) and Sec13/31 (12 µg)] (Aridor et al., 1998) for 30 min on ice (lane 1), or at 32°C (lanes 2–6) in the absence (lanes 1 and 2) or presence of 1-butanol (lane 3, 1%; lane 4, 2%) or tert-butanol (lane 5, 1%; lane 6, 2%). At the end of the incubation, the vesicle fraction (H for
h
igh speed pellet) was separated from the donor membranes (M for
m
edium speed pellet) by differential centrifugation, and the export of VSV-G into the vesicular fraction was determined by western blot analysis with anti-VSV-G antibody. The lower panel is a quantitative densitometry analysis of the amount of VSV-G in the vesicle fraction. Results are presented as a percentage of maximal budding under non-perturbed conditions (25% of total VSV-G in the starting membrane, lane 2). The experiment presented is representative of at least three independent experiments.
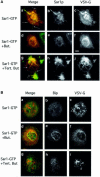
Fig. 3. PLD is required for export site formation. (A) PLD is required for Sar1p-induced tubule formation. VSV-infected NRK cells were permeabilized and incubated (Aridor et al., 2001) in the absence of cytosol in the presence of 9 µg of Sar1p-GTP (a–i), 1-butanol (d–f, 1.5%) or tert-butanol (g–i, 1.5%). The distribution of Sar1p (b, e and h) and VSV-G (c, f and i) was determined using indirect immunofluorescence (merged images are shown in a, d and g). Arrows in (a) indicate VSV-G-containing Sar1p-dependent tubules. Butanol inhibits tubule formation but does not block Sar1 binding (d–f). Tert-butanol does not inhibit the Sar1-dependent tubule formation (g–i; arrows in g indicate tubules on the ER; the bar in f is 5 µm. All images are representative of at least three independent experiments. (B) Cells were untreated or treated with butanol or tert-butanol as indicated (described above) and the localization of VSV-G (c, f and i) or Bip (b, e and h) was analyzed by confocal microscopy (merged images are shown in a, d and g).
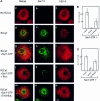
Fig. 4. PLD is required for COPII assembly. (A) PLD is required for COPII assembly and ER export: VSV-infected NRK cells were permeabilized and incubated as previously described (Plutner et al., 1992) in either the absence (a–c) or presence (d–f) of cytosol or cytosol and 9 µg of recombinant Sar1p-GTP (H79G mutation) (g–o), in the presence of 1-butanol (j–i) 1.5%) or tert-butanol (m–o 1.5%), in 220 ml for 30 min at 32°C. The distribution of VSV-G (c, f, i, l and o) and Sec13 (b, e, h, k and n) was determined using indirect immunofluorescence with specific antibodies (merged images are shown in a, d, g, j and m). Note the efficient mobilization of VSV-G to VTCs (compare c with f). In the presence of Sar1p-GTP (H79G), note the co-localization of Sec13 with VSV-G (arrows in g). Butanol (j–l) but not tert-butanol (m–o, see arrows indicating Sec 13 recruitment to VSV-G-containing export sites) inhibits COPII assembly and ER export (the bar in o is 5 µm). A representative experiment is shown; similar results were obtained in three separate experiments. (B) Analysis of the number of Sec13-positive sites per cell. Random fields of cells were captured and analyzed for Sec13-labeled sites/cell as described in Materials and methods. (C) Analysis of average area of Sec13-coated ER export sites. Random fields of cells were captured and the average area of Sec13 recruitment sites (in pixels) was determined as described in Materials and methods (averages ± SD are shown; control, n = 70; butanol n = 37; tert-butanol n = 67).
Fig. 5. PLD is required for Sec23/24 recruitment and ER export. (A) Butanol inhibits Sec23/24 recruitment. Membranes (lanes 1–9) were incubated with cytosol, in the absence (lane 1) or presence of Sar1p-GTP (lanes 2–10) and in the presence of increasing concentrations of 1-butanol (lanes 3–7) or tert-butanol (lane 8, 1%; lane 9, 2%) as indicated. Lane 10 is incubation with Sar1p-GTP cytosol and no membranes. At the end of the incubations, membranes were washed and the recruitment of Sec23/24 was determined by western blotting with a Sec23-specific antibody. Quantitative densitometry of the upper gel is shown in the lower panel. (B) 1-butanol does not inhibit Sar1 recruitment. Membranes (lanes 1–9) were incubated with cytosol, in the absence (lane 1) or presence of Sar1p-GTP (lanes 2–10; lane 10 is incubation without membranes) and in the presence of increasing concentrations of 1-butanol (lanes 3, 0.25%; 4, 0.5%; 5, 1%; 6, 1.5%; 7, 2%) or tert-butanol (lane 8, 1%; lane 9, 2%) as indicated. At the end of the incubations, the membranes were layered on a 15% sucrose cushion and collected by centrifugation (Aridor and Balch, 2000). Sar1p recruitment was analyzed by western blotting with specific antibody. (C) PLD is required to support Sar1p-dependent Sec23/24 recruitment. Membranes were pre-treated with buffer (control, lanes 1–6) or peanut PLD (40 U/ml) for 20 min at 32°C (lanes 8–13); lane 7 is incubation with no membranes. After incubation, the membranes were transferred to ice and collected by centrifugation. The membranes were resuspended and incubated in the presence of purified Sec23/24 subunits (lanes 1–13), in the absence (lanes 1 and 8) or presence of Sar1p-GTP (lanes 2–7 and lanes 9–13) and increasing concentrations of 1-butanol (lanes 3–6 and 10–13). After incubation, the membranes were collected and the recruitment of Sec23 was determined as described in (A). In the right panel, ribophorin II present in PLD-pre-treated or untreated membranes is shown. (D) Phosphatidic acid is required to support Sar1p-dependent Sec23/24 recruitment. Membranes were incubated on ice in the presence or absence of phosphatidic acid-containing liposomes at a final concentration of 100 µM for 30 min. At the end of incubation, the membranes were collected by centrifugation and incubated with cytosol in the absence (lanes 1 and 6) or presence (lanes 2–5 and 7–10) of Sar1p-GTP and increasing concentrations of butanol (lanes 3–5 and 8–10; 1, 1.5 and 2% butanol, respectively). After incubation, the membranes were collected and the recruitment of Sec23/24 was determined. (E) Phosphatidic acid is required for ER export. VSV-G-containing membranes were incubated on ice in the presence of ATP and the presence or absence of phosphatidic acid-containing liposomes at a final concentration of 10 µM for 30 min (stage 1). At the end of incubation, the membranes were collected by centrifugation and incubated with cytosol on ice or at 32°C in the presence or absence of 1% 1-butanol as indicated (stage 2). The vesicular fraction was separated and the export of VSV-G was determined by western blot.
Fig. 6. Sar1p activation stimulates PLD activity. (A) Membranes (75 µg) were incubated with ATP and GTP in the presence of recombinant Sar1p-GTP (H79G mutation) (8 µg), or recombinant Sar1p-GDP (T39N mutation) (8 µg), ethanol and radiolabeled liposomes for 60 min at 37°C. At the end of incubation, the lipids were extracted and the formation of phosphatidylethanol was determined using TLC (Iyer and Kusner, 1999). Results were averaged from two independent experiments and are presented as fold stimulation over control conditions set as 1. (B) Membranes (75 µg) were incubated with ATP in the presence of recombinant Sar1p-GTP (H79G mutation) (8 µg), recombinant myrARF1 (8 µg) or recombinant Sar1p-GDP (T39N mutation) (8 µg) in the presence or absence of GTP-γ-S (100 µM) for 30 min at 37°C as indicated. At the end of the incubation, the membranes were re-isolated by centrifugation, washed and resuspended in the presence of GTP-γ-S (100 µM provided to all samples), ethanol and radiolabeled liposomes, and further incubated for 60 min at 37°C. The lipids were then extracted and the formation of phosphatidylethanol was determined using TLC. Results were averaged from two independent experiments and are presented as fold stimulation over control conditions set as 1.
Similar articles
- Sar1p N-terminal helix initiates membrane curvature and completes the fission of a COPII vesicle.
Lee MC, Orci L, Hamamoto S, Futai E, Ravazzola M, Schekman R. Lee MC, et al. Cell. 2005 Aug 26;122(4):605-17. doi: 10.1016/j.cell.2005.07.025. Cell. 2005. PMID: 16122427 - Dissection of COPII subunit-cargo assembly and disassembly kinetics during Sar1p-GTP hydrolysis.
Sato K, Nakano A. Sato K, et al. Nat Struct Mol Biol. 2005 Feb;12(2):167-74. doi: 10.1038/nsmb893. Epub 2005 Jan 23. Nat Struct Mol Biol. 2005. PMID: 15665868 - Sed4p stimulates Sar1p GTP hydrolysis and promotes limited coat disassembly.
Kodera C, Yorimitsu T, Nakano A, Sato K. Kodera C, et al. Traffic. 2011 May;12(5):591-9. doi: 10.1111/j.1600-0854.2011.01173.x. Epub 2011 Feb 25. Traffic. 2011. PMID: 21291503 - A structural view of the COPII vesicle coat.
Bickford LC, Mossessova E, Goldberg J. Bickford LC, et al. Curr Opin Struct Biol. 2004 Apr;14(2):147-53. doi: 10.1016/j.sbi.2004.02.002. Curr Opin Struct Biol. 2004. PMID: 15093828 Review. - COPII: a membrane coat that forms endoplasmic reticulum-derived vesicles.
Barlowe C. Barlowe C. FEBS Lett. 1995 Aug 1;369(1):93-6. doi: 10.1016/0014-5793(95)00618-j. FEBS Lett. 1995. PMID: 7641893 Review.
Cited by
- A real-time, click chemistry imaging approach reveals stimulus-specific subcellular locations of phospholipase D activity.
Liang D, Wu K, Tei R, Bumpus TW, Ye J, Baskin JM. Liang D, et al. Proc Natl Acad Sci U S A. 2019 Jul 30;116(31):15453-15462. doi: 10.1073/pnas.1903949116. Epub 2019 Jul 16. Proc Natl Acad Sci U S A. 2019. PMID: 31311871 Free PMC article. - COPII and the regulation of protein sorting in mammals.
Zanetti G, Pahuja KB, Studer S, Shim S, Schekman R. Zanetti G, et al. Nat Cell Biol. 2011 Dec 22;14(1):20-8. doi: 10.1038/ncb2390. Nat Cell Biol. 2011. PMID: 22193160 Review. - Dopey1-Mon2 complex binds to dual-lipids and recruits kinesin-1 for membrane trafficking.
Mahajan D, Tie HC, Chen B, Lu L. Mahajan D, et al. Nat Commun. 2019 Jul 19;10(1):3218. doi: 10.1038/s41467-019-11056-5. Nat Commun. 2019. PMID: 31324769 Free PMC article. - Overexpression of Cu-Zn SOD in Brucella abortus suppresses bacterial intracellular replication via down-regulation of Sar1 activity.
Liu X, Zhou M, Yang Y, Wu J, Peng Q. Liu X, et al. Oncotarget. 2018 Jan 10;9(11):9596-9607. doi: 10.18632/oncotarget.24073. eCollection 2018 Feb 9. Oncotarget. 2018. PMID: 29515756 Free PMC article. - Diacylglycerol is required for the formation of COPI vesicles in the Golgi-to-ER transport pathway.
Fernández-Ulibarri I, Vilella M, Lázaro-Diéguez F, Sarri E, Martínez SE, Jiménez N, Claro E, Mérida I, Burger KN, Egea G. Fernández-Ulibarri I, et al. Mol Biol Cell. 2007 Sep;18(9):3250-63. doi: 10.1091/mbc.e07-04-0334. Epub 2007 Jun 13. Mol Biol Cell. 2007. PMID: 17567948 Free PMC article.
References
- Allan D. (1996) Mapping the lipid distribution in the membranes of BHK cells (mini-review). Mol. Membr. Biol., 13, 81–84. - PubMed
- Antonny B. and Schekman,R. (2001) ER export: public transportation by the COPII coach. Curr. Opin. Cell Biol., 13, 438–443. - PubMed
- Aridor M. and Balch,W.E. (2000) Kinase signaling initiates coat complex II (COPII) recruitment and export from the mammalian endoplasmic reticulum. J. Biol. Chem., 275, 35673–35676. - PubMed
- Aridor M. and Traub,L.M. (2002) Cargo selection in vesicular transport: the making and breaking of a coat. Traffic, 3, 537–546. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK54782/DK/NIDDK NIH HHS/United States
- DK02465/DK/NIDDK NIH HHS/United States
- R01 DK062318/DK/NIDDK NIH HHS/United States
- R01 DK054782/DK/NIDDK NIH HHS/United States
- DK51183/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases