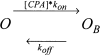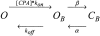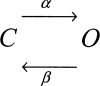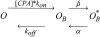Gating competence of constitutively open CLC-0 mutants revealed by the interaction with a small organic Inhibitor - PubMed (original) (raw)
Gating competence of constitutively open CLC-0 mutants revealed by the interaction with a small organic Inhibitor
Sonia Traverso et al. J Gen Physiol. 2003 Sep.
Abstract
Opening of CLC chloride channels is coupled to the translocation of the permeant anion. From the recent structure determination of bacterial CLC proteins in the closed and open configuration, a glutamate residue was hypothesized to form part of the Cl--sensitive gate. The negatively charged side-chain of the glutamate was suggested to occlude the permeation pathway in the closed state, while opening of a single protopore of the double-pore channel would reflect mainly a movement of this side-chain toward the extracellular pore vestibule, with little rearrangement of the rest of the channel. Here we show that mutating this critical residue (Glu166) in the prototype Torpedo CLC-0 to alanine, serine, or lysine leads to constitutively open channels, whereas a mutation to aspartate strongly slowed down opening. Furthermore, we investigated the interaction of the small organic channel blocker p-chlorophenoxy-acetic acid (CPA) with the mutants E166A and E166S. Both mutants were strongly inhibited by CPA at negative voltages with a >200-fold larger affinity than for wild-type CLC-0 (apparent KD at -140 mV approximately 4 micro M). A three-state linear model with an open state, a low-affinity and a high-affinity CPA-bound state can quantitatively describe steady-state and kinetic properties of the CPA block. The parameters of the model and additional mutagenesis suggest that the high-affinity CPA-bound state is similar to the closed configuration of the protopore gate of wild-type CLC-0. In the E166A mutant the glutamate side chain that occludes the permeation pathway is absent. Thus, if gating consists only in movement of this side-chain the mutant E166A should not be able to assume a closed conformation. It may thus be that fast gating in CLC-0 is more complex than anticipated from the bacterial structures.
Figures
Figure 1.
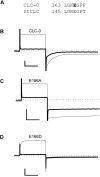
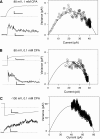
Phenotype of mutations. Glutamate 148 of the StCLC channel (Dutzler et al., 2002) marks the beginning of helix F and is part of a highly conserved structural motif of CLC channels. The phenotype of mutations E166A and E166D are illustrated in C and D. Mutant E166S (not depicted) was indistinguishable from mutant E166A, and mutant E166K (not depicted) was similar to E166A. voltage-clamp traces obtained from whole oocytes from the indicated constructs are shown. Single traces in 100 mM NaCl (thin trace) and 100 mM NaI (thick trace) measured from the same oocyte are shown superimposed. They were evoked by a pulse to 60 mV followed by a pulse to −140 mV. Horizontal bars, 50 ms. Vertical bars: 2 μA (B), 16 μA (C), 4 μA (D). Similar results were obtained for at least five oocytes for each mutant.
Figure 2.
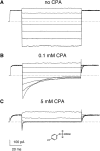
Inhibition of mutant E166A by CPA. Patch-clamp data from a representative inside-out patch are shown in the absence of CPA (A), and in the presence of 0.1 mM CPA (B) and 5 mM CPA (C). The pulse protocol consists of a prepulse to 80 mV, a test pulse to various potentials (the responses to −140, −100, −60, −20, 20, 60, and 100 mV are shown) and a “tail” pulse to 80 mV. The chemical structure of CPA is shown in C as an inset. Nearly identical results were obtained for the mutation E166S (not depicted).
Figure 3.
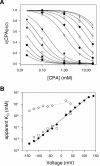
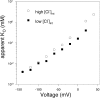
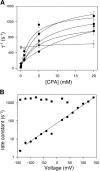
Steady-state inhibition by CPA. The inhibition seen at steady-state is shown in A as a function of [CPA] (filled circles, −140 mV; open circles, −120 mV; filled squares, −100 mV; open squares, −80 mV; filled triangles up, −60 mV, open triangles up, −40 mV; filled triangles down, −20 mV; open triangles down, 20 mV; filled diamonds, 40 mV; open diamonds, 60 mV; filled hexagon, 80 mV; open hexagon, 100 mV; cross, 120 mV; star, 140 mV). Data were obtained by averages from 5 patches. Lines in A are best fits of Eq. 1. The resulting KD app is shown in B (filled circles) as a function of voltage. The solid line represents a best exponential fit to the data from −120 to 120 mV of the form KD app(V) = KD app(0)*exp(z*V/(RT)) with a valence z = 0.96 and KD app(0) = 0.48 mM. Diamonds represent the open channel KD estimated by the fit of Eq. 3 to the relaxation rates shown in Fig. 8 A and the dotted line indicates an exponential function of the form KD (V) = KD (0)*exp(zO*V/(RT)) with zO = 0.23 and KD(0) = 11 mM. Open squares are calculated from Eq. 2 using the parameters obtained by fitting Eq. 3 to the relaxation rates (see Fig. 8).
Figure 4.
Block of E166A in low intracellular chloride. The steady-state block was evaluated using an intracellular solution in which 90 mM chloride was replaced by glutamate resulting in a solution with 14 mM Cl− (filled squares). For comparison, the data from Fig. 3 obtained in the regular Cl− solution are also shown (open circles). Data in low Cl− were obtained by the average from three patches.
Figure 5.
Nonstationary noise analysis of mutant E166A in the presence of CPA. Examples of nonstationary noise analysis are shown under different conditions from different patches (A, test pulse to 80 mV after a prepulse to −140 mV in the presence of 1 mM CPA; B, test pulse to 80 mV after a prepulse to −140 mV in the presence of 0.1 mM CPA; C, test pulse to −100 mV after a prepulse to 80 mV in the presence of 0.1 mM CPA). On the left are shown the mean (top trace) and the variance (bottom trace), while on the right the variance is plotted versus the mean (symbols) and fitted with a parabola (line) as described in
materials and methods
. The parameters obtained by the fit are, A, i = 0.37 pA, N = 123, pmax = 0.88; B, i = 0.40 pA, N = 96, pmax = 0.95; C, i = 0.91, N = 89, pmax = 0.94. Bars: 5 ms, 10 pA, 1 pA2 (A and B); 10 ms, 30 pA, 5 pA2 (C). Similar results were obtained in a total of four patches.
Figure 6.
Recordings from a patch with only a few channels. Representative traces from a single patch held at −100 mV (A–C) or 60 mV (E–G) in the absence of CPA (gray trace in A and E), with 0.1 mM CPA (black trace in A and F), or 5 mM CPA (B, C, and G) are shown. In C a short stretch of the trace in B is shown at a higher time-resolution (filtered at 500 Hz, while the longer traces were filtered at 200 Hz before display). In D a raw amplitude histogram of the recording at −100 mV in the presence of 0.1 mM CPA (gray trace) and 5 mM CPA (black trace) is shown. The vertical lines are drawn at current values of −0.8 and −0.61 pA to highlight the first nonzero peaks of the respective amplitude histograms. The bin-width for the amplitude histograms was 5 fA. The vertical scale bar in A also applies to B and C, and the scale bars in F also apply to E and G. The patch most likely contained two channels with four pores. Similar results were obtained in a total of four patches.
SCHEME III
Figure 7.
Single-exponential relaxations of mutant E166A in the presence of CPA. Examples of relaxations in the presence of CPA (A and B, 5 mM; C, 0.1 mM) at 80 mV (prepulse to −140 mV) (A), −120 mV (prepulse to 80 mV) (B), and −140 mV (prepulse to 80 mV) (C) are shown (thin lines) superimposed with single exponential fits (thick gray traces) (time constants, A, 2.2 ms; B, 2.7 ms; C, 17.5 ms).
Figure 8.
Kinetic analysis of CPA inhibition of mutant E166A. A shows relaxation rates obtained by fitting single exponential functions to the relaxations of the currents in the presence of CPA for exemplary voltages (circles, −140 mV; squares, −100 mV; triangles up, −60 mV; triangles down, −20 mV; diamond, 20 mV; open hexagons, 80 mV). Lines are fits of Eq. 3 to the data with the parameters α, β, and KD for each voltage. In the positive voltage-range essentially only the opening rate, α, could be determined because the affinity for CPA is too low, so that the second term in Eq. 3 is small compared with α. The resulting open channel KD is shown in Fig. 3 B (diamonds), while α (circles) and β (squares) are displayed in B as a function of voltage. The line in B is a function of the form α(V) = α(0)*exp(zα*V/(RT)) with zα = 0.58 and α(0) = 75 s−1.
SCHEME IV
SCHEME I
SCHEME V
Figure 9.
Effect of reduced [Cl−]ext on CPA block of E166A. The main graph shows the open-probability as a function of voltage of E166A channels with 0.1 mM intracellular CPA in 110 mM [Cl−]ext (circles) and 20 mM [Cl−]ext (squares) (n = 4 each). The inset shows a typical family of voltage-clamp traces measured from an outside-out patch in high (top traces) and low (bottom traces) [Cl−]ext. The asterisk indicates the constant tail pulse at −140 mV. At 0.1 mM CPA practically no inhibition is seen at voltages >80 mV and thus the normalized initial currents at the constant “tail” pulse directly reflect the apparent open-probability at the end of a previous conditioning pulse. The normalized initial current values at this test pulse were thus used to calculate the open-probability. The lines are fits of a Boltzmann function with a voltage of half-maximal activation of −71 mV (high Cl−) and −41 mV (low Cl−). Scale bars in the inset indicate 50 ms and 50 pA, respectively.
SCHEME VI
Figure 10.
Effect of additional mutations on the kinetics of CPA block of mutant E166A. Shown are representative current traces measured with 1 mM CPA for mutant E166A (thin black trace), mutant E166A/S123T (dashed gray trace), and mutant E166A/K519E (dashed black trace). None of the mutants show any relaxation in the absence of CPA. The currents are normalized to have similar amplitude at the 80-mV prepulse, that is followed by a pulse to −140 mV, and a repolarization to 80 mV. Note also the strong outward rectification of mutant E166A/K519E, compatible with the known alterations of the open channel properties of the mutant K519E (Pusch et al., 1995; Ludewig et al., 1997a). Similar results were obtained in a total of five patches for each mutant (E166A/S123T and E166A/K519E).
SCHEME II
SCHEME VII
Similar articles
- Conformational changes in the pore of CLC-0.
Accardi A, Pusch M. Accardi A, et al. J Gen Physiol. 2003 Sep;122(3):277-93. doi: 10.1085/jgp.200308834. Epub 2003 Aug 11. J Gen Physiol. 2003. PMID: 12913090 Free PMC article. - Mechanism of block of single protopores of the Torpedo chloride channel ClC-0 by 2-(p-chlorophenoxy)butyric acid (CPB).
Pusch M, Accardi A, Liantonio A, Ferrera L, De Luca A, Camerino DC, Conti F. Pusch M, et al. J Gen Physiol. 2001 Jul;118(1):45-62. doi: 10.1085/jgp.118.1.45. J Gen Physiol. 2001. PMID: 11432801 Free PMC article. - Blocking pore-open mutants of CLC-0 by amphiphilic blockers.
Zhang XD, Tseng PY, Yu WP, Chen TY. Zhang XD, et al. J Gen Physiol. 2009 Jan;133(1):43-58. doi: 10.1085/jgp.200810004. Epub 2008 Dec 15. J Gen Physiol. 2009. PMID: 19088381 Free PMC article. - Mechanisms of block of muscle type CLC chloride channels (Review).
Pusch M, Accardi A, Liantonio A, Guida P, Traverso S, Camerino DC, Conti F. Pusch M, et al. Mol Membr Biol. 2002 Oct-Dec;19(4):285-92. doi: 10.1080/09687680210166938. Mol Membr Biol. 2002. PMID: 12512775 Review. - Coupling gating with ion permeation in ClC channels.
Chen TY. Chen TY. Sci STKE. 2003 Jun 24;2003(188):pe23. doi: 10.1126/stke.2003.188.pe23. Sci STKE. 2003. PMID: 12824475 Review.
Cited by
- CLCNKB mutations causing mild Bartter syndrome profoundly alter the pH and Ca2+ dependence of ClC-Kb channels.
Andrini O, Keck M, L'Hoste S, Briones R, Mansour-Hendili L, Grand T, Sepúlveda FV, Blanchard A, Lourdel S, Vargas-Poussou R, Teulon J. Andrini O, et al. Pflugers Arch. 2014 Sep;466(9):1713-23. doi: 10.1007/s00424-013-1401-2. Epub 2013 Nov 24. Pflugers Arch. 2014. PMID: 24271511 - Extracellular determinants of anion discrimination of the Cl-/H+ antiporter protein CLC-5.
De Stefano S, Pusch M, Zifarelli G. De Stefano S, et al. J Biol Chem. 2011 Dec 23;286(51):44134-44144. doi: 10.1074/jbc.M111.272815. Epub 2011 Sep 15. J Biol Chem. 2011. PMID: 21921031 Free PMC article. - Review. Proton-coupled gating in chloride channels.
Lísal J, Maduke M. Lísal J, et al. Philos Trans R Soc Lond B Biol Sci. 2009 Jan 27;364(1514):181-7. doi: 10.1098/rstb.2008.0123. Philos Trans R Soc Lond B Biol Sci. 2009. PMID: 18957380 Free PMC article. Review. - Dynamical model of the CLC-2 ion channel reveals conformational changes associated with selectivity-filter gating.
McKiernan KA, Koster AK, Maduke M, Pande VS. McKiernan KA, et al. PLoS Comput Biol. 2020 Mar 30;16(3):e1007530. doi: 10.1371/journal.pcbi.1007530. eCollection 2020 Mar. PLoS Comput Biol. 2020. PMID: 32226009 Free PMC article. - Biophysical and Pharmacological Insights to CLC Chloride Channels.
Kwon HC, Fairclough RH, Chen TY. Kwon HC, et al. Handb Exp Pharmacol. 2024;283:1-34. doi: 10.1007/164_2022_594. Handb Exp Pharmacol. 2024. PMID: 35768555