Impaired differentiation of osteoclasts in TREM-2-deficient individuals - PubMed (original) (raw)
Impaired differentiation of osteoclasts in TREM-2-deficient individuals
Marina Cella et al. J Exp Med. 2003.
Abstract
TREM-2 is an immunoglobulin-like cell surface receptor associated with DAP12/KARAP that activates monocyte-derived dendritic cells (DCs) in vitro. Recently, it has been shown that genetic defects of human DAP12/KARAP and TREM-2 result in a rare syndrome characterized by bone cysts and presenile dementia called Nasu-Hakola disease. This observation suggests that TREM-2 may function in myeloid cells other than DCs, most probably osteoclasts (OCs) and microglial cells, which are involved in bone modeling and brain function. Consistent with this prediction, here we show that OC differentiation is dramatically arrested in TREM-2-deficient patients, resulting in large aggregates of immature OCs that exhibit impaired bone resorptive activity. These results demonstrate a critical role for TREM-2 in the differentiation of mononuclear myeloid precursors into functional multinucleated OCs.
Figures
Figure 1.
Monocytic precursors from normal donors express TREM-2 at early times of culture with M-CSF or GM-CSF and IL-4. TREM-2 is expressed within 48–72 h of culture with M-CSF or GM-CSF and IL-4, reaching a maximum level at days 3–4. It is worth noting that IL-4 alone is sufficient to induce TREM-2 expression, consistent with a possible role of Th2 cytokines in regulation of TREM-2.
Figure 2.
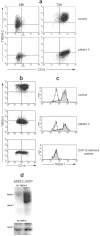
Patient A exhibits a selective lack of TREM-2 expression in monocytes cultured with M-CSF or GM-CSF and IL-4. (a) TREM-2 and CD16 expression in monocytes cultured for 48 or 72 h in the presence of M-CSF from normal or TREM-2–deficient patients. (b) TREM-2 and CD1b expression on control, TREM-2–deficient, and DAP12-deficient monocytes cultured for 4 d in GM-CSF and IL-4. (c) TREM-1 expression on freshly isolated monocytes from normal, TREM-2–deficient, and DAP12-deficient individuals. (d) Expression of TREM-2 and DAP12 proteins in normal and TREM-2–deficient monocyte-derived DCs at day 6 of culture assessed by immunoblot. Similar results were obtained with monocytes derived from patient B (not depicted).
Figure 3.
Arrest of differentiation, defect of actin polymerization, reduced levels of vitronectin receptor (VNR), and lack expression of calcitonin receptor (CTR) in OCs from TREM-2–deficient patients. (a–d) Images of OC cultures derived from normal (a and c) and TREM-2–deficient individuals (b and d). Images were taken on a Nikon phase contrast microscope with a 10DL objective. In c and d, cells were stained for intracellular TRAP. Upon stimulation with M-CSF, RANKL, and mAb 3.8B1, TREM-2–deficient monocytic precursors do not fuse, but form large aggregates of cells intensely positive for the TRAP reaction. Multinucleated cells with up to three nuclei were rarely detected in the OC cultures from TREM-2–deficient patients (red arrow). (e–h) Actin cytoskeleton of OCs from normal (e and g) and TREM-2–deficient individuals (f and h) generated in glass chamber slides, stained on day 10 of culture with Phalloidin-Oregon Green and analyzed by confocal microscopy (×20). (i) Content of F actin/cell was determined in control and TREM-2–deficient cells by flow cytometry 48 h after stimulation of monocytes with M-CSF, RANKL, and the 3.8B1 mAb. At this early time point immature OCs could still be recovered from culture wells. (j) Three-fold dilutions of cDNAs obtained from control OC cultures, control monocytes, or TREM-2–deficient OC cultures were amplified by RT-PCR with oligonucleotide pairs specific for vitronectin receptor (VNR), calcitonin receptor (CTR), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). PCR products were separated by agarose gel electrophoresis and visualized with ethidium bromide. Molecular weight markers are indicated.
Figure 4.
Impaired resorptive function of TREM-2–deficient immature OCs. Resorptive function of normal and TREM-2–deficient OCs on dentin slices (a–f). Monocytic precursors from healthy individuals (a and b) and TREM-2–deficient patients (c–f) were cultured on dentin slices. At day 10, dentin slices were stained with hematoxylin and inspected by light microscopy before (a, c, and e) and after (b, d, and f) cell removal. Resorption pits appear as dark spots stained with hematoxylin after cell removal. Normal monocytes differentiated into multinucleated OCs (a) that generated resorption pits (b). In general, TREM-2–deficient mononuclear immature OCs (c) did not excavate resorptive pits (d). Only a few pits were detected (f) that corresponded in number to the few multinucleated cells generated (e).
Figure 5.
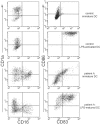
A subset of TREM-2–deficient DCs expresses the macro-phage marker CD16. Immature DCs from normal (top panels) and TREM-2–deficient individuals (bottom panels) were generated by culturing peripheral blood monocytes with GM-CSF and IL-4. At day 4, DCs were analyzed for expression of CD1a, CD16, CD83, and CD86. A population of CD1a− CD16+ macrophage-like cells was detected in the cultures of TREM-2–deficient patient A but not in control cultures. Identical results were obtained from DC cultures of patient B (not depicted). DC activation was monitored 24 h after stimulation with LPS. CD86 and CD83 were up-regulated on LPS-activated DCs in both TREM-2–deficient and control DC.
Similar articles
- DAP12/TREM2 deficiency results in impaired osteoclast differentiation and osteoporotic features.
Paloneva J, Mandelin J, Kiialainen A, Bohling T, Prudlo J, Hakola P, Haltia M, Konttinen YT, Peltonen L. Paloneva J, et al. J Exp Med. 2003 Aug 18;198(4):669-75. doi: 10.1084/jem.20030027. J Exp Med. 2003. PMID: 12925681 Free PMC article. - The enigmatic function of TREM-2 in osteoclastogenesis.
Colonna M, Turnbull I, Klesney-Tait J. Colonna M, et al. Adv Exp Med Biol. 2007;602:97-105. doi: 10.1007/978-0-387-72009-8_13. Adv Exp Med Biol. 2007. PMID: 17966394 Review. - TREM2, a DAP12-associated receptor, regulates osteoclast differentiation and function.
Humphrey MB, Daws MR, Spusta SC, Niemi EC, Torchia JA, Lanier LL, Seaman WE, Nakamura MC. Humphrey MB, et al. J Bone Miner Res. 2006 Feb;21(2):237-45. doi: 10.1359/JBMR.051016. Epub 2005 Oct 20. J Bone Miner Res. 2006. PMID: 16418779 - Blockade of TREM-2 exacerbates experimental autoimmune encephalomyelitis.
Piccio L, Buonsanti C, Mariani M, Cella M, Gilfillan S, Cross AH, Colonna M, Panina-Bordignon P. Piccio L, et al. Eur J Immunol. 2007 May;37(5):1290-301. doi: 10.1002/eji.200636837. Eur J Immunol. 2007. PMID: 17407101 - KARAP/DAP12/TYROBP: three names and a multiplicity of biological functions.
Tomasello E, Vivier E. Tomasello E, et al. Eur J Immunol. 2005 Jun;35(6):1670-7. doi: 10.1002/eji.200425932. Eur J Immunol. 2005. PMID: 15884055 Review.
Cited by
- Regulation of osteoclasts by membrane-derived lipid mediators.
Oikawa T, Kuroda Y, Matsuo K. Oikawa T, et al. Cell Mol Life Sci. 2013 Sep;70(18):3341-53. doi: 10.1007/s00018-012-1238-4. Epub 2013 Jan 8. Cell Mol Life Sci. 2013. PMID: 23296124 Free PMC article. Review. - Isoliquiritigenin Derivatives Inhibit RANKL-Induced Osteoclastogenesis by Regulating p38 and NF-κB Activation in RAW 264.7 Cells.
Jeong S, Lee S, Kim K, Lee Y, Lee J, Oh S, Choi JW, Kim SW, Hwang KC, Lim S. Jeong S, et al. Molecules. 2020 Aug 27;25(17):3908. doi: 10.3390/molecules25173908. Molecules. 2020. PMID: 32867185 Free PMC article. - Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2.
Takahashi K, Rochford CD, Neumann H. Takahashi K, et al. J Exp Med. 2005 Feb 21;201(4):647-57. doi: 10.1084/jem.20041611. J Exp Med. 2005. PMID: 15728241 Free PMC article. - Regulation of osteoclast function and bone mass by RAGE.
Zhou Z, Immel D, Xi CX, Bierhaus A, Feng X, Mei L, Nawroth P, Stern DM, Xiong WC. Zhou Z, et al. J Exp Med. 2006 Apr 17;203(4):1067-80. doi: 10.1084/jem.20051947. Epub 2006 Apr 10. J Exp Med. 2006. PMID: 16606672 Free PMC article. - The triggering receptor expressed on myeloid cells (TREM) in inflammatory bowel disease pathogenesis.
Genua M, Rutella S, Correale C, Danese S. Genua M, et al. J Transl Med. 2014 Oct 28;12:293. doi: 10.1186/s12967-014-0293-z. J Transl Med. 2014. PMID: 25347935 Free PMC article. Review.
References
- Bouchon, A., F. Facchetti, M.A. Weigand, and M. Colonna. 2001. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 410:1103–1107. - PubMed
- Daws, M.R., L.L. Lanier, W.E. Seaman, and J.C. Ryan. 2001. Cloning and characterization of a novel mouse myeloid DAP12-associated receptor family. Eur. J. Immunol. 31:783–791. - PubMed
- Chung, D.H., W.E. Seaman, and M.R. Daws. 2002. Characterization of TREM-3, an activating receptor on mouse macrophages: definition of a family of single Ig domain receptors on mouse chromosome 17. Eur. J. Immunol. 32:59–66. - PubMed
- Lanier, L.L., and A.B. Bakker. 2000. The ITAM-bearing transmembrane adaptor DAP12 in lymphoid and myeloid cell function. Immunol. Today. 21:611–614. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases