Extracellular signal-regulated kinase 7, a regulator of hormone-dependent estrogen receptor destruction - PubMed (original) (raw)
Extracellular signal-regulated kinase 7, a regulator of hormone-dependent estrogen receptor destruction
Lorin M Henrich et al. Mol Cell Biol. 2003 Sep.
Abstract
Estrogen receptor alpha (ER alpha) degradation is regulated by ubiquitination, but the signaling pathways that modulate ER alpha turnover are unknown. We found that extracellular signal-regulated kinase 7 (ERK7) preferentially enhances the destruction of ER alpha but not the related androgen receptor. Loss of ERK7 was correlated with breast cancer progression, and all ER alpha-positive breast tumors had decreased ERK7 expression compared to that found in normal breast tissue. In human breast cells, a dominant-negative ERK7 mutant decreased the rate of endogenous ER alpha degradation >4-fold in the presence of hormone and potentiated estrogen responsiveness. ERK7 targets the ER alpha ligand-binding domain for destruction by enhancing its ubiquitination. Thus, ERK7 is a novel regulator of estrogen responsiveness through its control of ER alpha turnover.
Figures
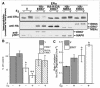
FIG. 1.
Active ERK7 enhances ERα destruction. (A) BHK cells were cotransfected with vectors encoding ERα and either the indicated kinase or vector control (V). The transfected cells were serum starved and treated with or without 10 nM estradiol (E2) for 6 h before addition of boiling sample buffer. Equal amounts of total protein were electrophoresed and immunoblotted. (B) Relative amounts of ERα were determined from the immunoblots by densitometry. The data are expressed as the percentage of ERα divided by ERα in the vector control in the absence of E2. The means ± standard errors are shown for n = 8. *, P < 0.05; **, P < 0.005 (Student's t test); values were obtained by comparing ERα levels with coexpressed ERK7 to those obtained with the appropriate vector control. (C) BHK cells were cotransfected with vectors encoding ERα and either HA-ERK7 or vector control (V). The transfected cells were then aliquoted and pretreated with 50 μM cycloheximide for 2 h before the addition of vehicle or 10 nM E2. Thereafter, at various time points over a 6-h period, the cells were lysed and immunoblotted and the relative amount of ERα was quantitated by densitometry. The rate data were fitted by using a single exponential decay, and the rate constants were determined. The means ± standard errors are shown for two separate experiments. *, P < 0.05 (Student's t test); value was obtained by comparing the rate constant to that of the appropriate control.
FIG. 2.
ERK7 specifically enhances the degradation of ERα. (A) BHK cells were cotransfected with vectors encoding ERα and either HA-ERK7 or vector control (V). The serum-starved, transfected cells were pretreated with or without 25 μM MG132 for 2 h before addition of vehicle or E2 and were then lysed after 6 h, and equal amounts of protein were electrophoresed and immunoblotted. (B) BHK cells were cotransfected with vectors encoding FLAG-IκBα and HA-ERK7 or HA-K43A-ERK7 or vector control (V). The serum-starved, transfected cells were treated with or without MG132 for 8 h and lysed, and equal amounts of protein were electrophoresed and immunoblotted. (C) BHK cells were cotransfected with vectors encoding HA-SF1 and HA-K43A-ERK7 or HA-ERK7 or vector control (V). The serum-starved, transfected cells were lysed, and equal amounts of protein were electrophoresed and immunoblotted. (D) BHK cells were cotransfected with vectors encoding ERα or HA-AR and HA-ERK7 or MYC-ERK7 or vector control (V). The transfected cells were then aliquoted and pretreated with 50 μM cycloheximide for 2 h before the addition of 10 nM E2 or 100 nM dihydroxytestosterone (DHT). Thereafter, at various times after ligand addition the cells were lysed and immunoblotted.
FIG. 3.
ERK7 is expressed in normal human breast cells. (A) In the left panel BHK cells were transfected with vectors encoding an HA-tagged wild type or a deletion mutant of ERK7 or vector control (V). The serum-starved, transfected cells were lysed, and equivalent amounts of protein were electrophoresed and immunoblotted. In the right panel normal human breast tissue was electrophoresed and immunoblotted with anti-ERK7 in the presence (+) or absence (−) of the antigenic peptide (200 μM) used to produce anti-ERK7 (p). (B) Equivalent amounts of lysate from serum-starved MCF-10A, MCF-7, and MDA-MB-231 cells were electrophoresed and immunoblotted. (C) Normal human breast tissue, benign tumor tissue, and breast cancer tissue samples were solubilized, normalized for Ran expression, electrophoresed, and immunoblotted. The tumor grade was obtained from the pathologist's report. ERα-positive samples are indicated by an asterisk.
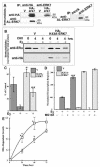
FIG. 4.
ERK7 regulates endogenous ERα degradation in human mammary cells. (A) In the left panel BHK cells were transfected with vector encoding HA-ERK7 or the vector control (V). The serum-starved, transfected cells were lysed, and the HA-ERK7 was immunoprecipitated (IP) with either anti-HA or anti-ERK7 antibodies. The IPs were electrophoresed and immunoblotted. In the right panel serum-starved MCF-7 cells were lysed and either immunoglobulin G or anti-ERK7 antibody was added to the lysate. The immunoprecipitates were electrophoresed and immunoblotted. (B) Serum-starved MCF-7 stable lines expressing either vector alone (V) or HA-K43A-ERK7 were immediately lysed after the addition of 50 μM cycloheximide (CHX) or after a 4-h pretreatment with CHX with or without 10 nM E2. Equivalent amounts of protein were electrophoresed and immunoblotted. (C) Relative ERα levels were determined by densitometry, and the data were expressed for each stable line as the percentage of ERα after incubation with CHX divided by ERα before incubation with CHX. The means ± standard errors for two separate experiments are shown. *, P < 0.05; **, P < 0.005 (Student's t test); values were obtained by comparing the response obtained with the lines expressing K43A-ERK7 to the appropriately treated control. (D) MCF-7 stables were cotransfected with an ERE-regulated luciferase reporter and β-galactosidase expression vectors. The serum-starved, transfected cells were treated with or without E2 and MG132. The luciferase and β-galactosidase activity was determined 24 h after the indicated treatments. The luciferase data were normalized to β-galactosidase activity to control for differences in transfection efficiency. To facilitate comparison between different experiments, the data were normalized so that, in the vector control, the response to vehicle addition was 0 and the response to E2 was 100. The means ± standard errors are shown (n = 3), each donein quadruplicate. **, P < 0.005 (Student's t test); this value was obtained by comparing the E2 response obtained with the vector control in the presence of MG132 to that obtained in its absence. (E) Stable MCF-7 lines were treated with or without 1 μM ICI 182,780. At various times the cells were lysed and growth was determined. ERα-dependent growth was obtained by subtracting the growth in ICI 182,780 from the growth obtained in vehicle control. The results are taken from two experiments in which each time point was determined in triplicate and from two independent lines. □, K43A-ERK7; ○, V. *, P < 0.05 (Student's t test); value was obtained by comparing the response obtained with the stable lines expressing HA-K43A-ERK7 to that found with the appropriately treated control.
FIG. 5.
Ser-118 phosphorylation does not influence ERα turnover. (A) BHK cells were cotransfected with vectors encoding ERα or S118A-ERα and HA-ERK7 or vector control (V). The serum-starved, transfected cells were treated with or without 10 nM E2 for 6 h before lysis. Lysates containing equivalent amounts of ERα were electrophoresed and immunoblotted. (B) BHK cells were cotransfected with vectors encoding S118-ERα and either HA-ERK7 or vector control (V). The transfected cells were then aliquoted and pretreated with 50 μM cycloheximide for 2 h before the addition of 10 nM E2. At various time points after E2 addition, the cells were lysed and immunoblotted and the relative amount of S118A-ERα was quantitated by densitometry. The rate constants were determined as described in the Fig. 1C legend, and the data shown are the means ± standard errors for two separate experiments.
FIG. 6.
ERK7 enhances the ubiquitination of the ERα ligand-binding domain. (A) BHK cells were cotransfected with vectors encoding a deletion mutant of ERα and either HA-ERK7 or vector control (V). The serum-starved, transfected cells were treated with or without 10 nM E2 for 6 h and were then lysed. Equal amounts of total protein were electrophoresed and immunoblotted. (B) BHK cells were transfected with a vector encoding HA-ubiquitin and either MYC-ERK7 or vector control (V). They were also transfected with either additional vector control or a vector encoding MYC-ERα(283-595). The transfected cells were pretreated with MG132 for 2 h before lysis. Immunoprecipitations (IPs) were performed by using anti-HA antibody, and the IPs were electrophoresed and immunoblotted (IB).
Similar articles
- The 26S proteasome is required for estrogen receptor-alpha and coactivator turnover and for efficient estrogen receptor-alpha transactivation.
Lonard DM, Nawaz Z, Smith CL, O'Malley BW. Lonard DM, et al. Mol Cell. 2000 Jun;5(6):939-48. doi: 10.1016/s1097-2765(00)80259-2. Mol Cell. 2000. PMID: 10911988 - ERK7 expression and kinase activity is regulated by the ubiquitin-proteosome pathway.
Kuo WL, Duke CJ, Abe MK, Kaplan EL, Gomes S, Rosner MR. Kuo WL, et al. J Biol Chem. 2004 May 28;279(22):23073-81. doi: 10.1074/jbc.M313696200. Epub 2004 Mar 19. J Biol Chem. 2004. PMID: 15033983 - Estrogen receptors and their downstream targets in cancer.
Ikeda K, Inoue S. Ikeda K, et al. Arch Histol Cytol. 2004 Dec;67(5):435-42. doi: 10.1679/aohc.67.435. Arch Histol Cytol. 2004. PMID: 15781984 Review. - Emerging roles of the ubiquitin-proteasome system in the steroid receptor signaling.
Lee JH, Lee MJ. Lee JH, et al. Arch Pharm Res. 2012 Mar;35(3):397-407. doi: 10.1007/s12272-012-0301-x. Epub 2012 Apr 5. Arch Pharm Res. 2012. PMID: 22477185 Review.
Cited by
- Mitogen-Activated Protein Kinase 15 Is a New Predictive Biomarker and Potential Therapeutic Target for Ovarian Cancer.
Zhong QH, Lau ATY, Xu YM. Zhong QH, et al. Int J Mol Sci. 2023 Dec 20;25(1):109. doi: 10.3390/ijms25010109. Int J Mol Sci. 2023. PMID: 38203280 Free PMC article. - Extracellular-Signal Regulated Kinase: A Central Molecule Driving Epithelial-Mesenchymal Transition in Cancer.
Olea-Flores M, Zuñiga-Eulogio MD, Mendoza-Catalán MA, Rodríguez-Ruiz HA, Castañeda-Saucedo E, Ortuño-Pineda C, Padilla-Benavides T, Navarro-Tito N. Olea-Flores M, et al. Int J Mol Sci. 2019 Jun 13;20(12):2885. doi: 10.3390/ijms20122885. Int J Mol Sci. 2019. PMID: 31200510 Free PMC article. Review. - Extracellular signal-regulated kinase 8 (ERK8) controls estrogen-related receptor α (ERRα) cellular localization and inhibits its transcriptional activity.
Rossi M, Colecchia D, Iavarone C, Strambi A, Piccioni F, Verrotti di Pianella A, Chiariello M. Rossi M, et al. J Biol Chem. 2011 Mar 11;286(10):8507-8522. doi: 10.1074/jbc.M110.179523. Epub 2010 Dec 28. J Biol Chem. 2011. PMID: 21190936 Free PMC article. - MAPK15 upregulation promotes cell proliferation and prevents DNA damage in male germ cell tumors.
Rossi M, Colecchia D, Ilardi G, Acunzo M, Nigita G, Sasdelli F, Celetti A, Strambi A, Staibano S, Croce CM, Chiariello M. Rossi M, et al. Oncotarget. 2016 Apr 12;7(15):20981-98. doi: 10.18632/oncotarget.8044. Oncotarget. 2016. PMID: 26988910 Free PMC article. - IGF1R and MAPK15 Emerge as Potential Targets of Pentabromobenzylisothioureas in Lung Neuroendocrine Neoplasms.
Motylewska E, Braun M, Kazimierczuk Z, Ławnicka H, Stępień H. Motylewska E, et al. Pharmaceuticals (Basel). 2020 Oct 29;13(11):354. doi: 10.3390/ph13110354. Pharmaceuticals (Basel). 2020. PMID: 33138224 Free PMC article.
References
- Alarid, E. T., N. Bakopoulos, and N. Solodin. 1999. Proteasome-mediated proteolysis of estrogen receptor: a novel component in autologous down-regulation. Mol. Endocrinol. 13:1522-1534. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases