Yaf9, a novel NuA4 histone acetyltransferase subunit, is required for the cellular response to spindle stress in yeast - PubMed (original) (raw)
Yaf9, a novel NuA4 histone acetyltransferase subunit, is required for the cellular response to spindle stress in yeast
Ivan Le Masson et al. Mol Cell Biol. 2003 Sep.
Abstract
Yaf9 is one of three proteins in budding yeast containing a YEATS domain. We show that Yaf9 is part of a large complex and that it coprecipitates with three known subunits of the NuA4 histone acetyltransferase. Although Esa1, the catalytic subunit of NuA4, is essential for viability, we found that yaf9 Delta mutants are viable but hypersensitive to microtubule depolymerizing agents and synthetically lethal with two different mutants of the mitotic apparatus. Microtubules depolymerized more readily in the yaf9Delta mutant compared to the wild type in the presence of nocodazole, and recovery of microtubule polymerization and cell division from limiting concentrations of nocodazole was inhibited. Two other NuA4 mutants (esa1-1851 and yng2 Delta) and nonacetylatable histone H4 mutants were also sensitive to benomyl. Furthermore, wild-type budding yeast were more resistant to benomyl when grown in the presence of trichostatin A, a histone deacetylase inhibitor. These results strongly suggest that acetylation of histone H4 by NuA4 is required for the cellular resistance to spindle stress.
Figures
FIG. 1.
Yaf9(Ynl107) interacts with Mps2 in a two-hybrid screen, contains a YEATS domain, and shows most similarity to the family of Gas41-like sequences in metazoans. (A) Yaf9, Spc24, and Bbp1 interact with Mps2 in a two-hybrid screen. The black arrows indicate two-hybrid interactions, and the red arrows indicate synthetic lethality between yaf9Δ and either bbp1-1 or spc24-11 mutants (see Fig. 3A). (B) Schematic representation of the Yaf9 sequence showing the location of the N-terminal YEATS domain and the minimal C-terminal 40-amino-acid sequence interacting with Mps2 in the two-hybrid screen. This C-terminal region contains a predicted 30-amino-acid coiled-coil sequence (gray bar) as detected by the COILS program (41) (
http://www.ch.embnet.org/software/COILS\_form.html
). V124 and K186 indicate valine-124 and lysine-186 of the Yaf9 sequence. (C) CLUSTALW alignments (26) of YEATS domains found in proteins similar to Yaf9. Shown are YEATS domains from Saccharomyces cerevisiae (S.c.) Yaf9 (Ynl107), Sas5, and Taf14, Schizosaccharomyces pombe (S.p.) SPAC17G8.07, Drosophila melanogaster (D.m.) CG9207, Caenorhabditis elegans (C.e.) Gfl-1, and Homo sapiens (H.s.) Gas41 and AF9. This analysis shows that Yaf9 and the Gas41 family of proteins contain YEATS domains that are more similar to each other than the YEATS domains found in the human AF9 and the yeast Taf14 and Sas5 proteins. The amino acid color code is as follows: yellow for proline, beige for glycine, purple for acidic residues, orange for basic residues, green for polar residues, light blue for hydrophobic residues, and dark blue for aromatic residues. An asterisk above the column of aligned amino acids indicates 100% identity, two dots indicate highly conserved amino acids, and a single dot indicates similar amino acids.
FIG. 2.
HCA indicates that Yaf9 is more similar to human (H.s.) Gas41 than to human AF9 or two other YEATS-domain proteins in S. cerevisiae (Taf14 and Sas5). HCA is a visual method that predicts protein secondary structure (9, 37). The linear one-dimensional sequence of the protein is written on an alpha-helix displayed along a cylinder. The cylinder is then cut parallel to its axis and unrolled in a bidimensional diagram. This diagram is duplicated in order to restore the full environment of each amino acid. The contours of clusters of hydrophobic residues are then drawn. This process has been automated by an online server (
http://smi.snv.jussieu.fr/hca/hca-form.html
). The alpha-helical net offers the best correspondence between the positions of hydrophobic clusters and regular secondary structures (alpha-helix and β strand). The HCA program uses the standard one-letter code for amino acids except for proline (regular secondary structure breaker), glycine (the least constrained amino acid), and serine and threonine (which can be accommodated in either hydrophilic or hydrophobic environments). Sequence features that were found in similar positions in Yaf9 and the other YEATS domain proteins were colored as follows: common hydrophobic clusters are in green, common nonhydrophobic amino acids are in yellow, positively charged amino” acids are in blue, and negatively charged amino acids are in red. “α” and “β” indicate the position of alpha-helical and β-sheet secondary structures for Yaf9 as predicted by the HCA analysis. Blue brackets show the positions of predicted coiled-coil sequences in the C-terminal regions of Yaf9, Gas41, and AF9. The global analysis of the sequences shows that these proteins are composed of two domains: a similar N-terminal region that corresponds to the YEATS domain and a C-terminal region that is more divergent. For Yaf9, Taf14, and AF9, a linker region could be clearly seen between these two domains. Examination of the HCA plots of human Gas41, AF9 and S. cerevisiae Yaf9, Taf14, and Sas5 sequences reveals the presence of similar hydrophobic motifs in the YEATS domain. The boundaries of this domain in the different proteins are shown with a dotted green line. Residues 55 to 100 of the Yaf9 sequence indicate an extremely conserved region shown within the dotted red lines. This central part of the first domain of the proteins was found to be characteristic of the YEATS domain family. The CLUSTALW (Fig. 1C) and HCA analyses show that the yeast Yaf9 and the human Gas41 YEATS domains are most similar to each other, whereas the yeast Taf14 and Sas5 YEATS domains are more similar to each other than either is to the remaining proteins. The second part of these proteins is more divergent and no similarity (>30%) in primary sequence could be found. Furthermore, the HCA profiles of Sas5, Taf14, and Yaf9 are different. On the other hand, similar hydrophobic motifs are found for the second domain of Yaf9 and Gas41. First, there are two hydrophobic clusters (green) separated by a conserved proline. Some amino acids, in particular charged residues, around this motif are well conserved. Second, a long alpha-helix predicted to form a coiled coil (blue brackets) is found at the C terminus of both Yaf9 and Gas41. We noted, however, that the charge distributions around these coiled coils are different, suggesting that their binding specificity may have diverged. Human AF9, both by its length (more than twice the length of the other YEATS proteins shown here) and by the profiling of its domains, seems the least similar of all of the YEATS proteins shown here.
FIG. 3.
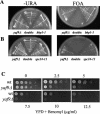
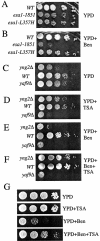
yaf9Δ is synthetically lethal with SPB and kinetochore mutants and it is hyper-sensitive to benomyl. (A) yaf9Δ is synthetically lethal with the bbp1-1 mutant. yaf9Δ (ILM162), bbp1-1 (YCS64), and yaf9Δ bbp1-1 (ILM113) cells containing the BBP1 URA3 plasmid (pli36) were streaked at 24°C, the permissive temperature for the bbp1-1 mutant, onto plates without uracil (−URA) or onto plates containing 5-FOA to counterselect the URA3 plasmid. The absence of growth on the 5-FOA plate of the yaf9Δ bbp1-1 (ILM113) cells (double in the figure) shows that the yaf9Δ bbp1-1 double mutant cannot grow in the absence of the BBP1 URA3 plasmid. (B) yaf9Δ is synthetically lethal with the spc24-11 mutant. yaf9Δ (ILM162), spc24-11 (ILM128), and yaf9Δ spc24-11 (ILM140) cells containing the SPC24 URA3 plasmid (pli39) were streaked at 24°C, the permissive temperature for the spc24-11 mutant, onto plates without uracil (−URA) or onto plates containing 5-FOA to counterselect the URA3 plasmid. The absence of growth on the 5-FOA plate of the yaf9Δ spc24-11 (ILM140) cells (double in the figure) shows that the yaf9Δ spc24-11 double mutant cannot grow in the absence of the SPC24 URA3 plasmid. (C) The yaf9Δ mutant is hypersensitive to benomyl. Wild-type (YPH499) and yaf9Δ (ILM162) cells at the same cell density in YPD were spotted in 10-fold serial dilutions onto YPD plates containing the indicated concentrations of benomyl, and the plates were subsequently incubated at 24°C for 3 days. WT, wild type.
FIG. 4.
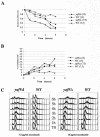
yaf9Δ mutants are hypersensitive to nocodazole, but they do not show significant defects in cell cycle arrest. (A to C) Wild-type (YPH499) and yaf9Δ (ILM162) mutants growing exponentially in YPD were treated with 7.5 or 15 μg of nocodazole/ml for the indicated periods of time at 30°C, and the fraction of viable cells (A) and the percentage of rebudded cells (cells with two buds rather than one) (B) were determined at each of the indicated times. (C) An aliquot of cells was also fixed for flow cytometry to determine their DNA content. WT, wild type.
FIG. 5.
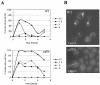
MTs depolymerize more readily and recover more slowly from limiting nocodazole in the yaf9Δ mutant compared to the wild type. Wild-type (CMY1228) and yaf9Δ cells (CMY1229) expressing GFP-Tub1 were grown in YPD at 30°C and treated with nocodazole at the concentrations and for the time periods indicated in the figure. (A) The percentage of wild-type and yaf9Δ cells with no detectable GFP-Tub1 foci after treatment with the indicated concentrations of nocodazole was quantified. A fluorescent GFP-Tub1 focus is found at the SPB of cells containing polymerized tubulin. The absence of such foci is thus an indication of an essentially complete depolymerization of MTs. (B) Images illustrating the different responses of wild-type and yaf9Δ cells to incubation with 3 μg of nocodazole/ml for 3 h. WT, wild type.
FIG. 6.
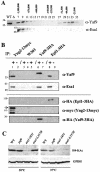
Yaf9 is in a large-molecular-weight complex, coprecipitates with the Esa1, Epl1, and Yng2 subunits of the NuA4 HAT complex but is not required for global histone H4 acetylation by NuA4. (A) A whole-cell protein extract from the wild-type strain YPH499 was chromatographed on a Superdex-200 gel filtration column. Fractions were collected and analyzed by SDS-PAGE and immunoblotting with anti-Yaf9 and anti-Esa1 antibodies. The numbers above each lane indicate the fractions and the molecular weight of marker proteins eluting in those fractions. Thenumbers to the left of the immunoblot indicate the molecular weight of precolored marker proteins. WT indicates a lane containing 40 μg of protein extract from the wild-type strain, and Δ indicates a lane containing 40 μg of protein extract from a yaf9Δ mutant. (B) Yaf9 coprecipitates with Esa1, Epl1, and Yng2. Extracts prepared from the wild-type W303-1a and from strains expressing Yng2-13myc (JCY1258), Yaf9-3HA (ILM49), or Epl1-3HA (JCY1259) were used for IPs with anti-myc or anti-HA monoclonal antibodies. The immunoprecipitates were electrophoresed on SDS-polyacrylamide gels, transferred to membranes, and probed consecutively with anti-Yaf9, anti-Esa1, anti-HA, and anti-myc antibodies in order to test for coprecipitation of NuA4 subunits and to verify the efficiency of IP of each tagged protein. Lane 1, anti-myc IP from extract of JCY1258 (Yng2-13myc) showing coimmunoprecipitation of Yaf9 and Esa1 with Yng2-13myc; lane 2, mock IP without primary antibody from JCY1258 (Yng2-13myc); lane 3, anti-myc IP from extract of untagged W303-1a; lane 4, mock IP without primary antibody from W303-1a; lane 5, anti-HA IP from extract of untagged W303-1a; lane 6, anti-HA IP from extract of ILM49 (Yaf9-3HA) showing coimmunoprecipitation of Esa1 with Yaf9-3HA; lane 7, mock IP without primary antibody from ILM49 (Yaf9-3HA); lane 8, anti-HA IP from extract of JCY1259 (Epl1-3HA) showing coimmunoprecipitation of Yaf9 and Esa1 with Epl1-3HA; lane 9, mock IP without primary antibody from JCY1259 (Epl1-3HA). The numbers to the left of each membrane show the molecular masses (in kilodaltons) of precolored protein markers. The arrow indicates Yng2-13myc. The two faster-migrating bands are proteolytic fragments produced during the IP incubations. (C) Immunoblots of whole-cell protein extracts from wild-type (W303-1a), yaf9Δ (ILM63), esa1-1851, and esa1-L357H cells grown at log phase in YPD at 30°C or transferred from 30 to 37°C for 4 h. The membranes were probed with anti-hyperacetylated histone H4 (Penta) rabbit antibodies (H4-Kac) from Upstate Biotechnology (catalog no. 06-946) and then reprobed with antibodies to glucose-6-phosphate dehydrogenase (GPDH) to verify the loading of equal amounts of protein. WT, wild type.
FIG. 7.
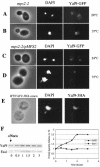
Yaf9 is a nuclear protein whose levels increase in response to spindle stress. (A to D) Yeast cells were observed by fluorescence microscopy to localize Yaf9-GFP with regard to nuclear DNA stained with DAPI. No signal was detectable with the GFP filter set for the parental control strain that did not express Yaf9-GFP. (A and B) mps2-2 YAF9-GFP (ILM74) cells grown at 24°C (A) or at 37°C (B) for 3 h; (C and D)mps2-2 YAF9-GFP/pMPS2 (ILM74/pli23) cells grown at 24°C (C) or at 37°C (D) for 3 h. (E) Wild-type/Yaf9-3HA cells (ILM49) growing in YPD at 30°C were treated with 15 μg of nocodazole/ml for 3 h. Cells were then fixed for immunofluorescence with anti-HA monoclonal antibodies, and nuclear DNA was stained with DAPI. No immunofluorescent signal was visible for the parental control strain that did not express Yaf9-3HA. (F) Western blot showing the levels of Yaf9 and Esa1 in whole-cell protein extracts prepared from wild-type cells (YPH499) treated with 15 μg of nocodazole/ml in YPD at 30°C for the indicated periods of time. The Yaf9 and Esa1 protein bands were quantified with a Fluorochem Imager. WT, wild type.
FIG. 8.
Identification of genes whose expression is altered in the yaf9Δ mutant (ILM162) relative to the wild type (YPH499) by whole-genome microarray analysis. (A) List of genes whose expression is increased at least 2.5-fold or decreased at least 3-fold in the wild type versus yaf9Δ for cells treated with 15 μg of nocodazole/ml for 3 h. (B) Verification by RT-PCR of genes whose expression is inhibited in the yaf9Δ mutant versus the wild type for cells growing exponentially in YPD or after treatment with 15 μg of nocodazole/ml for 3 h.
FIG. 8.
Identification of genes whose expression is altered in the yaf9Δ mutant (ILM162) relative to the wild type (YPH499) by whole-genome microarray analysis. (A) List of genes whose expression is increased at least 2.5-fold or decreased at least 3-fold in the wild type versus yaf9Δ for cells treated with 15 μg of nocodazole/ml for 3 h. (B) Verification by RT-PCR of genes whose expression is inhibited in the yaf9Δ mutant versus the wild type for cells growing exponentially in YPD or after treatment with 15 μg of nocodazole/ml for 3 h.
FIG. 9.
The esa1-1851 and yng2Δ mutants are hypersensitive to benomyl, and the HDAC inhibitor TSA differentially affects the growth and benomyl resistance of wild type and NuA4 HAT mutants. (A and B) Wild type (W303-1a) and esa1-1851 (MSY2431) and esa1-L357H (MSY2432) mutants were spotted onto either YPD alone or YPD plus 15 μg of benomyl/ml at 30°C for 3 days. (C to F) Tenfold serial dilutions of exponentially growing yng2Δ (CMY1237), wild-type (W303-1a), and yaf9Δ (ILM63) cells in YPD at the same cell density were spotted onto plates containing the indicated media and incubatedat 24°C for 1 day (A and B) or 4 days (C and D). YPD+TSA is YPD containing TSA at 30 μg/ml, YPD+Ben is YPD containing benomyl at 10 μg/ml, YPD+Ben+TSA is YPD containing benomyl at 10 μg/ml and TSA at 30 μg/ml. (G) Wild-type (W303-1a) cells were spotted on the indicated media and incubated at 24°C for 2 days. WT, wild type.
FIG. 10.
Nonacetylatable histone H4 mutants are hypersensitive to benomyl. Wild-type and mutant cells at the same cell density in YPD were spotted in 10-fold serial dilutions onto YPD plates without benomyl (YPD) or with 15 μg of benomyl/ml (Ben), followed by incubation at 28°C for 3 days. For each mutant allele, the amino acid substitutions at positions 5, 8, 12, and 16 or the N-terminal tail are indicated as K (lysine), Q (glutamine), or R (arginine). Allele hhf1-25 contains an insertion of G-K-G at position 3 of the hhf1-10 tail (designated QQQQ), whereas allele hhf1-35 contains an insertion of G-K-G at position 12 of the hhf1-10 tail (designated QQQQ). Both hhf1-10 and hhf1-34 are roughly 5 orders of magnitude hypersensitive to benomyl and are the only mutants that lack any acetylatable lysine in their histone H4 tail.
Similar articles
- The Yaf9 component of the SWR1 and NuA4 complexes is required for proper gene expression, histone H4 acetylation, and Htz1 replacement near telomeres.
Zhang H, Richardson DO, Roberts DN, Utley R, Erdjument-Bromage H, Tempst P, Côté J, Cairns BR. Zhang H, et al. Mol Cell Biol. 2004 Nov;24(21):9424-36. doi: 10.1128/MCB.24.21.9424-9436.2004. Mol Cell Biol. 2004. PMID: 15485911 Free PMC article. - Multiple links between the NuA4 histone acetyltransferase complex and epigenetic control of transcription.
Galarneau L, Nourani A, Boudreault AA, Zhang Y, Héliot L, Allard S, Savard J, Lane WS, Stillman DJ, Côté J. Galarneau L, et al. Mol Cell. 2000 Jun;5(6):927-37. doi: 10.1016/s1097-2765(00)80258-0. Mol Cell. 2000. PMID: 10911987 - NuA4 subunit Yng2 function in intra-S-phase DNA damage response.
Choy JS, Kron SJ. Choy JS, et al. Mol Cell Biol. 2002 Dec;22(23):8215-25. doi: 10.1128/MCB.22.23.8215-8225.2002. Mol Cell Biol. 2002. PMID: 12417725 Free PMC article. - Reading chromatin: insights from yeast into YEATS domain structure and function.
Schulze JM, Wang AY, Kobor MS. Schulze JM, et al. Epigenetics. 2010 Oct 1;5(7):573-7. doi: 10.4161/epi.5.7.12856. Epub 2010 Oct 1. Epigenetics. 2010. PMID: 20657183 Free PMC article. Review. - Histone acetylation and deacetylation in yeast.
Kurdistani SK, Grunstein M. Kurdistani SK, et al. Nat Rev Mol Cell Biol. 2003 Apr;4(4):276-84. doi: 10.1038/nrm1075. Nat Rev Mol Cell Biol. 2003. PMID: 12671650 Review.
Cited by
- ING Proteins: Tumour Suppressors or Oncoproteins.
Jacquet K, Binda O. Jacquet K, et al. Cancers (Basel). 2021 Apr 27;13(9):2110. doi: 10.3390/cancers13092110. Cancers (Basel). 2021. PMID: 33925563 Free PMC article. Review. - Catching Nucleosome by Its Decorated Tails Determines Its Functional States.
Sehrawat P, Shobhawat R, Kumar A. Sehrawat P, et al. Front Genet. 2022 Jul 14;13:903923. doi: 10.3389/fgene.2022.903923. eCollection 2022. Front Genet. 2022. PMID: 35910215 Free PMC article. Review. - Small Molecules Targeting the Specific Domains of Histone-Mark Readers in Cancer Therapy.
Zhu H, Wei T, Cai Y, Jin J. Zhu H, et al. Molecules. 2020 Jan 29;25(3):578. doi: 10.3390/molecules25030578. Molecules. 2020. PMID: 32013155 Free PMC article. Review. - Regulation of Cell Proliferation and Migration by miR-203 via GAS41/miR-10b Axis in Human Glioblastoma Cells.
Pal D, Mukhopadhyay D, Ramaiah MJ, Sarma P, Bhadra U, Bhadra MP. Pal D, et al. PLoS One. 2016 Jul 28;11(7):e0159092. doi: 10.1371/journal.pone.0159092. eCollection 2016. PLoS One. 2016. PMID: 27467502 Free PMC article. - SWI/SNF recruitment to a DNA double-strand break by the NuA4 and Gcn5 histone acetyltransferases.
Bennett G, Peterson CL. Bennett G, et al. DNA Repair (Amst). 2015 Jun;30:38-45. doi: 10.1016/j.dnarep.2015.03.006. Epub 2015 Mar 25. DNA Repair (Amst). 2015. PMID: 25869823 Free PMC article.
References
- Bilbao-Cortes, D., M. Hetzer, G. Langst, P. B. Becker, and I. W. Mattaj. 2002. Ran binds to chromatin by two distinct mechanisms. Curr. Biol. 12:1151-1156. - PubMed
- Bird, A. W., D. Y. Yu, M. G. Pray-Grant, Q. Qiu, K. E. Harmon, P. C. Megee, P. A. Grant, M. M. Smith, and M. F. Christman. 2002. Acetylation of histone H4 by Esa1 is required for DNA double-strand break repair. Nature 419:411-415. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases