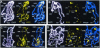Protein phosphatase 2A activity affects histone H3 phosphorylation and transcription in Drosophila melanogaster - PubMed (original) (raw)
Protein phosphatase 2A activity affects histone H3 phosphorylation and transcription in Drosophila melanogaster
Scott J Nowak et al. Mol Cell Biol. 2003 Sep.
Abstract
Transcriptional activation of the heat shock genes during the heat shock response in Drosophila has been intimately linked to phosphorylation of histone H3 at serine 10, whereas repression of non-heat-shock genes correlates with dephosphorylation of histone H3. It is then possible that specific kinase and/or phosphatase activities may regulate histone phosphorylation and therefore transcription activation and repression, respectively. We find that treatment of cells with strong phosphatase inhibitors interferes with the genome-wide dephosphorylation of histone H3 normally observed at non-heat-shock genes during heat shock. Mutants in protein phosphatase type 2A (PP2A) also display reduced genome-wide H3 dephosphorylation, and sites of H3 phosphorylation that do not contain heat shock genes remain transcriptionally active during heat shock in PP2A mutants. Finally, the SET protein, a potent and highly selective inhibitor of PP2A activity that inhibits PP2A-mediated dephosphorylation of Ser10-phosphorylated H3, is detected at transcriptionally active regions of polytene chromosomes. These results suggest that activation and repression of gene expression during heat shock might be regulated by changes in PP2A activity controlled by the SET protein.
Figures
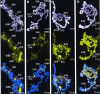
FIG. 1.
Effect of okadaic acid on histone H3 phosphorylation during heat shock. Salivary glands isolated from wild-type larvae were incubated in Grace's medium containing 0 nM (A), 10 nM (B), 50 nM (C), and 100 nM (D) okadaic acid. All salivary glands were heat shocked at 37°C for 20 min before squashing, immunostained for Ser10-phosphorylated histone H3 (FITC, yellow), and counterstained with DAPI (white). Merged images are a composite of both channels shown, antibody staining in yellow and DAPI counterstaining in blue. In all photographs, identifiable heat shock loci are labeled; although not all heat shock genes are visible in these photographs, data shown in other figures indicate that all contain Ser10-phosphorylated histone H3. Comparison of treated with untreated chromosomes indicates that okadaic acid prevents the genome-wide dephosphorylation of Ser10-phosphorylated histone H3 isoforms during heat shock.
FIG. 2.
Protein phosphatase type 2A dephosphorylates Ser10-phosphorylated histone H3 both in vivo and in vitro. (A) Purified core histones containing Ser10-phosphorylated histone H3 were incubated with purified PP2A in the presence or absence of 50 nM okadaic acid as indicated. The PP2A enzyme strongly dephosphorylates Ser10-phosphorylated histone H3. Catalytic activity of PP2A is strongly inhibited by incubation with 50 nM okadaic acid. (B) Whole-larval extracts were prepared from wild-type and twsP mutant larvae that either were not heat shocked (0) or were heat shocked for 20 min (20), resolved on an SDS-15% polyacrylamide gel, and immunostained for the presence of the Ser10-phosphorylated isoform of histone H3. The twsP mutant extracts contained a greater concentration of Ser10-phosphorylated histone H3 than wild-type controls both before and after heat shock.
FIG. 3.
Comparison of histone H3 phosphorylation in wild-type (A and B) versus twsP (C and D) mutant larvae. Wild-type and mutant larvae were heat shocked for 0 min (not heat shocked) (A and C) or 10 min (B and D). Polytene chromosomes were prepared and immunostained for Ser10-phosphorylated histone H3 (FITC, yellow) and counterstained with DAPI (white) to view the DNA. Merged images are a composite of both channels shown, with antibody staining (FITC) in yellow and DAPI counterstaining in blue. Prominent ecdysone gene-containing loci are marked in non-heat-shocked chromosomal spreads (A and C). In heat-shocked samples, visible heat shock loci are labeled, and asterisks indicate regions of strong labeling that do not contain heat shock genes (B and D). After a 10-min heat shock, puffing and phosphorylation are observed only at the heat shock loci in wild-type larvae (B). By contrast, numerous loci that do not contain heat shock genes are phosphorylated in twsP mutants during heat shock (D).
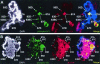
FIG. 4.
Non-heat-shock genes are transcribed in twsP mutants after heat shock. Salivary glands isolated from wild-type (A) and twsP mutant (B) larvae were heat shocked for 20 min at 37°C in Grace's medium containing bromouridine (BrUTP). Polytene chromosomes were then immunostained for the presence of BrUTP (red) and Ser10-phosphorylated histone H3 (phospho-H3, green). Chromosome spreads were also counterstained with DAPI (white) to view the DNA. DAPI counterstain information is represented as blue in merged images. Visible heat shock loci are indicated in all panels. Strong regions of labeling that do not contain heat shock genes are indicated with an asterisk. Merged images indicate that numerous non-heat-shock gene loci containing the Ser10-phosphorylated histone H3 isoform in twsP mutants are also actively transcribed after heat shock.
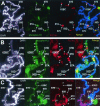
FIG. 5.
Sites that contain Ser10-phosphorylated histone H3 isoform also contain active RNA polymerase II during heat shock. Polytene chromosomes were prepared from wild-type (A) and twsP mutant (B) larvae. In addition, salivary glands from wild-type larvae were treated with 50 nM okadaic acid (C). All samples were heat shocked for 20 min at 37°C, immunostained with antibodies against hyperphosphorylated RNA Pol II (Pol IIo, red) and the Ser10-phosphorylated histone H3 isoform (phospho-H3, green), and counterstained with DAPI (white) to view the DNA. DAPI counterstain information is represented as blue in merged images. Visible heat shock gene-containing loci are labeled in all panels. The active form of RNA polymerase II was observed at all sites containing the Ser10-phosphorylated H3 isoform, including the heat shock gene loci (A,B, and C). Additionally, prominent Pol IIo labeling was detected at regions that do not contain heat shock genes (indicated with asterisks) in twsP mutant (B) and okadaic acid-treated (C) chromosomes.
FIG. 6.
The SET oncoprotein inhibits PP2A-mediated dephosphorylation of phosphohistone H3 and is present at transcriptionally active loci. (A) Purified human core histones containing Ser10-phosphorylated histone H3 (lane 1) were incubated with purified human PP2A (lane 2). The presence of 1 μl of in vitro reticulocyte lysate-translated SET is capable of inhibiting PP2A-mediated dephosphorylation of histone H3 (lane 4). Translation reactions that did not contain SET protein showed minimal inhibition of PP2A activity (lane 3). (B) Antibodies to the SET gene product were generated and used to detect SET in whole Drosophila pupa extracts (lane 1) and the in vitro-translated SET protein expressed via a reticulocyte lysate system and utilized in a PP2A activity assay (lanes 3 and 4). Lane 3 contains 5 μl of reticulocyte lysate that does not contain the expressed SET protein. Lane 4 contains 5 μl of reticulocyte lysate containing expressed SET. Lane 2 shows the Coomassie staining of lane 1. Lanes 5 and 6 show Coomassie staining of lanes 3 and 4, respectively. Molecular mass size standards (in kilodaltons) are indicated at right. (C,D, and E) Genomic distribution of SET protein in polytene chromosomes prepared from wild-type larvae before (C) and after (E) heat shock and immunostained for the presence of SET (SET, red) and the Ser10-phosphorylated histone H3 isoform (phospho-H3, green). Chromosome spreads were also counterstained with DAPI (white) to view the DNA. DAPI counterstain information is represented as blue in merged images. Before heat shock, the SET protein is detected at puffed and H3-phosphorylated (transcriptionally active) loci, including the ecdysone-regulated genes, which are labeled (C). In situ hybridization with a fragment of the hsp70 transcription unit (hsp70, green) reveals that prior to heat shock, the SET protein (SET, red) does not colocalize with the hsp70 genes (D). During heat shock, strong SET staining is also detected at the actively transcribing heat shock puffs (E).
Similar articles
- RNA polymerase II-mediated transcription at active loci does not require histone H3S10 phosphorylation in Drosophila.
Cai W, Bao X, Deng H, Jin Y, Girton J, Johansen J, Johansen KM. Cai W, et al. Development. 2008 Sep;135(17):2917-25. doi: 10.1242/dev.024927. Epub 2008 Jul 30. Development. 2008. PMID: 18667461 Free PMC article. - Phosphorylation of histone H3 at Ser10 facilitates RNA polymerase II release from promoter-proximal pausing in Drosophila.
Ivaldi MS, Karam CS, Corces VG. Ivaldi MS, et al. Genes Dev. 2007 Nov 1;21(21):2818-31. doi: 10.1101/gad.1604007. Epub 2007 Oct 17. Genes Dev. 2007. PMID: 17942706 Free PMC article. - Involvement of histone H3 (Ser10) phosphorylation in chromosome condensation without Cdc2 kinase and mitogen-activated protein kinase activation in pig oocytes.
Bui HT, Yamaoka E, Miyano T. Bui HT, et al. Biol Reprod. 2004 Jun;70(6):1843-51. doi: 10.1095/biolreprod.103.026070. Epub 2004 Feb 11. Biol Reprod. 2004. PMID: 14960481 - Phosphorylation of histone H3 correlates with transcriptionally active loci.
Nowak SJ, Corces VG. Nowak SJ, et al. Genes Dev. 2000 Dec 1;14(23):3003-13. doi: 10.1101/gad.848800. Genes Dev. 2000. PMID: 11114889 Free PMC article.
Cited by
- Antagonistic roles of Drosophila Tctp and Brahma in chromatin remodelling and stabilizing repeated sequences.
Hong ST, Choi KW. Hong ST, et al. Nat Commun. 2016 Sep 30;7:12988. doi: 10.1038/ncomms12988. Nat Commun. 2016. PMID: 27687497 Free PMC article. - The chromodomain-containing NH(2)-terminus of Chromator interacts with histone H1 and is required for correct targeting to chromatin.
Yao C, Ding Y, Cai W, Wang C, Girton J, Johansen KM, Johansen J. Yao C, et al. Chromosoma. 2012 Apr;121(2):209-20. doi: 10.1007/s00412-011-0355-4. Epub 2011 Dec 28. Chromosoma. 2012. PMID: 22203189 Free PMC article. - Protein phosphatase 2A enables expression of interleukin 17 (IL-17) through chromatin remodeling.
Apostolidis SA, Rauen T, Hedrich CM, Tsokos GC, Crispín JC. Apostolidis SA, et al. J Biol Chem. 2013 Sep 13;288(37):26775-84. doi: 10.1074/jbc.M113.483743. Epub 2013 Aug 5. J Biol Chem. 2013. PMID: 23918926 Free PMC article. - Akirin links twist-regulated transcription with the Brahma chromatin remodeling complex during embryogenesis.
Nowak SJ, Aihara H, Gonzalez K, Nibu Y, Baylies MK. Nowak SJ, et al. PLoS Genet. 2012;8(3):e1002547. doi: 10.1371/journal.pgen.1002547. Epub 2012 Mar 1. PLoS Genet. 2012. PMID: 22396663 Free PMC article. - H2Av facilitates H3S10 phosphorylation but is not required for heat shock-induced chromatin decondensation or transcriptional elongation.
Li Y, Wang C, Cai W, Sengupta S, Zavortink M, Deng H, Girton J, Johansen J, Johansen KM. Li Y, et al. Development. 2017 Sep 15;144(18):3232-3240. doi: 10.1242/dev.151134. Epub 2017 Aug 14. Development. 2017. PMID: 28807902 Free PMC article.
References
- Adachi, Y., G. N. Pavlakis, and T. D. Copeland. 1994. Identification of in vivo phosphorylation sites of SET, a nuclear phosphoprotein encoded by the translocation breakpoint in acute undifferentiated leukemia. FEBS Lett. 340:231-235. - PubMed
- Adler, H. T., F. S. Nallaseth, G. Walter, and D. C. Tkachuk. 1997. HRX leukemic fusion proteins form a heterocomplex with the leukemia-associated protein SET and protein phosphatase 2A. J. Biol. Chem. 272:28407-28414. - PubMed
- Ahmad, K., and S. Henikoff. 2002. The histone variant h3.3 marks active chromatin by replication-independent nucleosome assembly. Mol. Cell 9:1191-1200. - PubMed
- Ajiro, K., H. Yasuda, and H. Tsuji. 1996. Vanadate triggers the transition from chromosome condensation to decondensation in a mitotic mutant (tsTM13) inactivation of p34cdc2/H1 kinase and dephosphorylation of mitosis-specific histone H3. Eur. J. Biochem. 241:923-930. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases