ZIP kinase triggers apoptosis from nuclear PML oncogenic domains - PubMed (original) (raw)
ZIP kinase triggers apoptosis from nuclear PML oncogenic domains
Taro Kawai et al. Mol Cell Biol. 2003 Sep.
Abstract
PML oncogenic domains (PODs), also referred to as nuclear dot 10 bodies, Kreb's bodies, or nuclear bodies, represent nuclear structures implicated in the regulation of a variety of cellular processes, including transcription, tumor suppression, and apoptosis. ZIP kinase (ZIPK) is a proapoptotic protein kinase with homology to DAP kinase, a protein kinase implicated in apoptosis. We show here that ZIPK is present in PODs, where it colocalizes with and binds to proapoptotic protein Daxx. Arsenic trioxide (As(2)O(3)) and gamma interferon (IFN-gamma), which accentuate POD formation, increased the association of ZIPK with PODs. In contrast, the kinase-inactive ZIPK resides in nuclei with a diffuse pattern and significantly prevents the association of Daxx with PODs, implying that ZIPK recruits Daxx to PODs via its catalytic activity. ZIPK also binds and phosphorylates proapoptotic protein Par-4. Association of ZIPK with Daxx was enhanced by coexpression of Par-4. Activation of caspases and induction of apoptosis were also observed in cells overexpressing these proteins. Conversely, small-interfering RNA-mediated reduction of ZIPK, Daxx, or Par-4 expression decreased activation of caspase and apoptosis induced by As(2)O(3) and IFN-gamma. These results suggest that ZIPK, in collaboration with Daxx and Par-4, mediates a novel nuclear pathway for apoptosis.
Figures
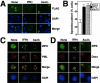
FIG. 1.
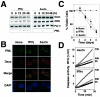
ZIPK colocalizes with Daxx in PODs. (A) HeLa cells grown on coverslips were transiently transfected with FLAG-ZIPK. After 36 h of transfection, cells were stimulated with 1,500 U of IFN-γ/ml or 1.0 μM As2O3 for 12 h. After fixation, cells were stained with an anti-FLAG antibody (M2) for the detection of ZIPK and DAPI for visualization of nuclei. Antibody detection was achieved with a FITC-conjugated anti-mouse antibody (green), followed by microscopy analysis. The bottom panels show the overlay result. (B) HeLa cells transiently transfected with FLAG-ZIPK were stimulated with IFN-γ and As2O3 for 12 h and stained with an anti-FLAG antibody, and the percentage of transfected cells with a speckled pattern was determined by counting a minimum of 200 transfected cells. Data represent means ± standard deviations (n = 3). (C and D) HeLa cells transiently transfected with FLAG-ZIPK were stimulated with IFN-γ or As2O3 for 12 h and stained sequentially with an anti-FLAG monoclonal antibody (top), an anti-PML polyclonal antibody (C, upper middle), or an anti-Daxx polyclonal antibody (D, upper middle) and DAPI (bottom). Antibody detection was achieved with a FITC-conjugated anti-mouse antibody (green) and a rhodamine-conjugated anti-rabbit antibody (red). Overlay results (merge) demonstrate colocalization of FLAG-ZIPK and PML or Daxx in response to IFN-γ and As2O3.
FIG. 2.
ZIPK associates with Daxx. (A) One million 293T cells were transiently transfected with 2.0 μg of plasmid DNA encoding FLAG-tagged Daxx. After 36 h, cell lysates were immunoprecipitated (IP) with control rabbit anti-mouse Ig serum, anti-ZIPK (Ab1), or another anti-ZIPK antibody (Ab2). Proteins were separated by SDS-PAGE, transferred to nitrocellulose, and blotted with an anti-FLAG monoclonal antibody (top). Aliquots of whole-cell lysates (WCL) were simultaneously subjected to immunoblot analysis using an anti-FLAG antibody (bottom). WB, Western blotting. (B) 293T cells were transiently transfected with the indicated combinations of HA-Daxx (FL), HA-Daxx ΔC (ΔC), FLAG-ZIPK, and FLAG-Tpl2. Total amounts of plasmids were kept constant at 6.0 μg by supplementation with the empty pcDNA3 plasmid. After 36 h, cell lysates were immunoprecipitated with an anti-HA monoclonal antibody, followed by Western blotting with an anti-FLAG antibody (top). Aliquots of whole-cell lysates were simultaneously subjected to immunoblot analysis using an anti-FLAG (middle) or anti-HA antibody (bottom). (C) HeLa cells (3 × 108) were stimulated with IFN-γ or As2O3 for 12 h. Cell lysates were prepared, and immunoprecipitated with a monoclonal Daxx or control (CNTL) antibody. The immunoprecipitates were separated by SDS-PAGE, followed by blotting with an anti-ZIPK antibody (Ab1). Aliquots of the whole-cell lysates were simultaneously subjected to Western blot analysis using an anti-Daxx or anti-ZIPK antibody as indicated. N, nonstimulated; I, IFN-γ; A, As2O3.
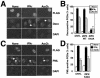
FIG. 3.
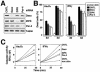
ZIPK K42A prevents Daxx translocation into PODs in response to IFN-γ and As2O3. (A) HeLa cells were transiently transfected with FLAG-ZIPK K42A. After 36 h of transfection, cells were stimulated with As2O3 or IFN-γ for 6 or 18 h, respectively. The cells were fixed and stained sequentially with an anti-FLAG antibody (top), an anti-Daxx antibody (middle), and DAPI (bottom). Arrows, cells expressing FLAG-ZIPK K42A. Note that Daxx translocation to PODs was prevented by expression of ZIPK K42A. (B) Cells were prepared as for panel A. The percentage of transfected cells with Daxx translocation to PODs is presented, counting at least 200 transfected cells from three independent experiments (means ± standard deviations [SD]). CNTL, control; WT, wild type. (C) HeLa cells were transiently transfected with FLAG-ZIPK K42A. After 36 h of transfection, cells were stimulated with As2O3 or IFN-γ for 6 or 18 h, respectively. The cells were fixed and stained sequentially with an anti-FLAG antibody (top), an anti-PML antibody (middle), and DAPI (bottom). Arrows, cells expressing FLAG-ZIPK K42A. Note that PML translocation to PODs was not affected by expression of ZIPK K42A. (D) Cells were prepared as for panel C. Shown are the percentages of cells with PML translocation to PODs, counting at least 200 transfected cells from three independent experiments (means ± SD).
FIG. 4.
ZIPK binds and phosphorylates Par-4. (A) Cell lysates prepared from HeLa cells (3 × 108) were divided into equal volumes and used for immunoprecipitation (IP) with an anti-Par-4 monoclonal antibody (P) or control IgG (C), followed by immunoblotting with an anti-ZIPK antibody (Ab1). Aliquots of whole-cell lysates (W) were simultaneously subjected to immunoblot analysis using an anti-ZIPK antibody. WB, Western blotting. Arrowhead, band corresponding to ZIPK. (B) Plasmids expressing ZIPK or mutant versions of it fused to the GAL4 DNA-binding domain were cotransformed with a plasmid expressing Par-4 or various mutant proteins fused to the GAL4 _trans_-activation domain. Interactions were detected on the basis of the ability of cells to grow on medium lacking leucine, tryptophan, and histidine (+). β-Gal, β-galactosidase. (C) COS-7 cells were transiently transfected with the indicated combinations of plasmids encoding FLAG-ZIPK, Myc-wild-type Par-4 (WT), Myc-Par-4 ΔLZ (ΔLZ), or Myc-Par-4 LZ (LZ), holding the amounts of DNA at 5.0 μg by addition of an empty vector. Cell lysates were immunoprecipitated with an anti-Myc antibody (9E10), followed by immunoblot analysis using an anti-FLAG antibody (M2) as indicated. (D) COS-7 cells were transiently transfected with the indicated combinations of plasmids. After 36 h, cell lysates were immunoprecipitated with an anti-Myc antibody and subjected to in vitro kinase assays. Aliquots of whole-cell lysates were simultaneously subjected to immunoblot analysis using an anti-FLAG or anti-Myc antibody. IVK, in vitro kinase assay. (E) HeLa cells grown on coverslips were transiently transfected with Myc-Par-4. At 36 h after transfection, cells were stained with an anti-Myc monoclonal antibody. Antibody detection was achieved by using a FITC-conjugated anti-mouse antibody.
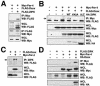
FIG. 5.
Par-4 facilitates the association between ZIPK and Daxx. (A) 293T cells (106 cells) were transiently transfected with 2.0 μg of Myc-Par-4 together with 2.0 μg of FLAG-Daxx or FLAG-ZIPK. Cell lysates were immunoprecipitated (IP) with an anti-Myc or anti-FLAG antibody, followed by immunoblot analysis using an anti-FLAG or anti-Myc antibody, as indicated. WB, Western blotting; WCL, whole-cell lysates. (B) 293T cells (106 cells) were transiently transfected with the indicated combinations of plasmids encoding Myc-Par-4, FLAG-Daxx, HA-wild-type (WT) ZIPK, HA-ZIPK K42A, or HA-ZIPK ΔLZ. Total amounts of DNA were kept at 6.0 μg. Cell lysates were immunoprecipitated with an anti-Myc antibody, followed by immunoblot analysis using an anti-FLAG antibody (top). Aliquots of whole-cell lysates were simultaneously subjected to immunoblot analysis using an anti-Myc (upper middle), anti-FLAG (lower middle), or anti-HA (bottom) antibody. (C) 293T cells (106 cells) were transiently transfected with 2.5 μg of expression plasmids encoding Myc-Par-4 and FLAG-Daxx as indicated. Total amounts of DNA were held at 5.0 μg by addition of an empty vector. After 36 h, cells were lysed and immunoprecipitated with an anti-ZIPK antibody (Ab1), followed by immunoblot analysis using an anti-FLAG antibody. Aliquots of the whole-cell lysates were simultaneously subjected to immunoblot analysis using an anti-FLAG or anti-Myc antibody. (D) 293T cells (106 cells) were transiently transfected with the indicated combinations of plasmids encoding FLAG-ZIPK, Myc-Par-4, and HA-Daxx, holding total amounts of DNA at 6.0 μg by addition of an empty vector. Cell lysates were immunoprecipitated with an anti-Myc antibody, followed by immunoblot analysis using an anti-FLAG antibody. Aliquots of whole-cell lysates were simultaneously subjected to immunoblot analysis using an anti-Myc, anti-FLAG, or anti-HA antibody.
FIG. 6.
Induction of cell death by ZIPK, Daxx, and Par-4. (A) 293T cells (5 × 105 cells) were transiently cotransfected with the indicated combinations of plasmids (total, 3.0 μg) together with 0.3 μg of a GFP expression plasmid. After 48 h, GFP-positive cells were evaluated by microscopy, and the percentages of GFP-positive cells with apoptotic morphology were determined, counting 200 cells (means ± standard deviations [SD]; n = 3). (B) 293T cells were transiently cotransfected with the indicated combinations of plasmids (total, 3.0 μg). After 48 h, cytoplasmic proteins were extracted and caspase activity was measured, with Ac-DEVD-AFC as the substrate. Data represent relative fluorescence units (RFU) normalized for protein concentration (means ± SD; n = 3). (C) 293T cells (5 × 105 cells) were transiently cotransfected with the indicated combinations of plasmids (total, 3.0 μg) together with 0.3 μg of GFP expression plasmids. After 48 h, the percentages of GFP-positive apoptotic cells were determined, counting 200 cells (means ± SD; n = 3).
FIG. 7.
As2O3 and IFN-γ induce POD formation and apoptosis. (A) Immunoblot analysis of ZIPK, Par-4, and Daxx in HeLa cells was performed using cell lysates normalized for total protein content (50 μg per lane) and specific antibodies that detect the endogenous proteins. Cells were stimulated for the indicated times with 1,500 U of IFN-γ/ml or 1.0 μM As2O3 prior to lysis. (B) HeLa cells stimulated for 12 h with IFN-γ or As2O3 were fixed and stained with an anti-PML monoclonal antibody and a FITC-conjugated anti-mouse IgG secondary antibody (top) and then stained with an anti-Daxx polyclonal antibody and a rhodamine-conjugated anti-rabbit secondary antibody (upper middle) and DAPI (bottom). Overlay results (merge) demonstrate colocalization of PML and Daxx in response to IFN-γ and As2O3. Cells were imaged by fluorescence microscopy. (C) HeLa cells were treated with IFN-γ and As2O3 for the indicated periods. The percentage (Å) of viable cells was determined by DAPI staining, counting a minimum of 300 cells (means ± standard errors; n = 3). (D) HeLa cells were stimulated with As2O3 or IFN-γ for 48 or 72 h. Cytoplasmic proteins were extracted, and the caspase activity was measured, with Ac-DEVD-AFC as the substrate. RFU, relative fluorescence units.
FIG. 8.
Dominant-negative ZIPK and Par-4 suppress apoptosis induced by POD-stimulating agents. 293T (5 × 105) cells were transiently cotransfected with the indicated plasmids (total, 3.0 μg), together with 0.3 μg of the GFP expression plasmid. After 12 h, cells were stimulated with 1,500 U of IFN-γ/ml, 1.0 μM As2O3, or 100 μM STS. After 3 days (IFN-γ and As2O3) or 24 h (STS) of incubation, the percentages of GFP-positive apoptotic cells were determined, counting 200 cells (means ± standard deviations; n = 3).
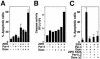
FIG. 9.
ZIPK, Daxx, and Par-4 are necessary for apoptosis mediated by As2O3 and IFN-γ, as determined by using siRNA. (A) HeLa cells were transfected with siRNA targeting ZIPK, Daxx, or Par-4, and cells were analyzed by immunoblotting using anti-ZIPK, anti-Daxx, anti-Par-4, and anti-tubulin antibodies, verifying siRNA-mediated reduction in endogenous ZIPK, Daxx, and Par-4. CNTL, control. (B) HeLa cells were treated with siRNA. After 24 h, cells were stimulated with IFN-γ or As2O3 for 3 or 4 days. The percentages of apoptotic cells were determined by DAPI staining, counting a minimum of 300 cells (means ± standard errors; n = 3). (C) HeLa cells were treated with siRNA. After 24 h, cells were stimulated with IFN-γ or As2O3 for 4 days. Extracts prepared from HeLa cells or after treatment with siRNA were tested for the activation of caspase 3, based on the release of AFC from the Ac-DEVD-AFC substrate.
Similar articles
- Human Daxx regulates Fas-induced apoptosis from nuclear PML oncogenic domains (PODs).
Torii S, Egan DA, Evans RA, Reed JC. Torii S, et al. EMBO J. 1999 Nov 1;18(21):6037-49. doi: 10.1093/emboj/18.21.6037. EMBO J. 1999. PMID: 10545115 Free PMC article. - Daxx silencing sensitizes cells to multiple apoptotic pathways.
Chen LY, Chen JD. Chen LY, et al. Mol Cell Biol. 2003 Oct;23(20):7108-21. doi: 10.1128/MCB.23.20.7108-7121.2003. Mol Cell Biol. 2003. PMID: 14517282 Free PMC article. - PML nuclear bodies and apoptosis.
Takahashi Y, Lallemand-Breitenbach V, Zhu J, de Thé H. Takahashi Y, et al. Oncogene. 2004 Apr 12;23(16):2819-24. doi: 10.1038/sj.onc.1207533. Oncogene. 2004. PMID: 15077145 Review. - PML NBs (ND10) and Daxx: from nuclear structure to protein function.
Lindsay CR, Morozov VM, Ishov AM. Lindsay CR, et al. Front Biosci. 2008 May 1;13:7132-42. doi: 10.2741/3216. Front Biosci. 2008. PMID: 18508722 Review.
Cited by
- ZIPK: a unique case of murine-specific divergence of a conserved vertebrate gene.
Shoval Y, Pietrokovski S, Kimchi A. Shoval Y, et al. PLoS Genet. 2007 Oct;3(10):1884-93. doi: 10.1371/journal.pgen.0030180. Epub 2007 Sep 7. PLoS Genet. 2007. PMID: 17953487 Free PMC article. - DAXX in cancer: phenomena, processes, mechanisms and regulation.
Mahmud I, Liao D. Mahmud I, et al. Nucleic Acids Res. 2019 Sep 5;47(15):7734-7752. doi: 10.1093/nar/gkz634. Nucleic Acids Res. 2019. PMID: 31350900 Free PMC article. Review. - Par-4: a new activator of myosin phosphatase.
Vetterkind S, Lee E, Sundberg E, Poythress RH, Tao TC, Preuss U, Morgan KG. Vetterkind S, et al. Mol Biol Cell. 2010 Apr 1;21(7):1214-24. doi: 10.1091/mbc.e09-08-0711. Epub 2010 Feb 3. Mol Biol Cell. 2010. PMID: 20130087 Free PMC article. - New modularity of DAP-kinases: alternative splicing of the DRP-1 gene produces a ZIPk-like isoform.
Shoval Y, Berissi H, Kimchi A, Pietrokovski S. Shoval Y, et al. PLoS One. 2011 Mar 8;6(2):e17344. doi: 10.1371/journal.pone.0017344. PLoS One. 2011. PMID: 21408167 Free PMC article. - Adenovirus type 5 early region 1B 55K oncoprotein-dependent degradation of cellular factor Daxx is required for efficient transformation of primary rodent cells.
Schreiner S, Wimmer P, Groitl P, Chen SY, Blanchette P, Branton PE, Dobner T. Schreiner S, et al. J Virol. 2011 Sep;85(17):8752-65. doi: 10.1128/JVI.00440-11. Epub 2011 Jun 22. J Virol. 2011. PMID: 21697482 Free PMC article.
References
- Camandola, S., and M. P. Mattson. 2000. Pro-apoptotic action of PAR-4 involves inhibition of NF-κB activity and suppression of BCL-2 expression. J. Neurosci. Res. 61:134-139. - PubMed
- Cardone, M. H., N. Roy, H. R. Stennicke, G. S. Salvesen, T. F. Franke, E. Stanbridge, S. Frisch, and J. C. Reed. 1998. Regulation of cell death protease caspase-9 by phosphorylation. Science 282:1318-1320. - PubMed
- Chang, H., H. Nishitoh, X. Yang, H. Ichijo, and D. Baltimore. 1998. Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein daxx. Science 281:1860-1863. - PubMed
- Cohen, O., and A. Kimchi 2001. DAP-kinase: from functional gene cloning to establishment of its role in apoptosis and cancer. Cell Death Differ. 8:6-15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials