Indian hedgehog and beta-catenin signaling: role in the sebaceous lineage of normal and neoplastic mammalian epidermis - PubMed (original) (raw)
. 2003 Sep 30;100 Suppl 1(Suppl 1):11873-80.
doi: 10.1073/pnas.1834202100. Epub 2003 Aug 13.
Affiliations
- PMID: 12917489
- PMCID: PMC304101
- DOI: 10.1073/pnas.1834202100
Indian hedgehog and beta-catenin signaling: role in the sebaceous lineage of normal and neoplastic mammalian epidermis
C Niemann et al. Proc Natl Acad Sci U S A. 2003.
Abstract
In mammalian epidermis, the level of beta-catenin signaling regulates lineage selection by stem cell progeny. High levels of beta-catenin stimulate formation of hair follicles, whereas low levels favor differentiation into interfollicular epidermis and sebocytes. In transgenic mouse epidermis, overexpression of beta-catenin leads to formation of hair follicle tumors, whereas overexpression of N-terminally truncated Lef1, which blocks beta-catenin signaling, results in spontaneous sebaceous tumors. Accompanying overexpression of beta-catenin is up-regulation of Sonic hedgehog (SHH) and its receptor, Patched (PTCH/Ptch). In DeltaNLef1 tumors Ptch mRNA is up-regulated in the absence of SHH. We now show that PTCH is up-regulated in both human and mouse sebaceous tumors and is accompanied by overexpression of Indian hedgehog (IHH). In normal sebaceous glands IHH is expressed in differentiated sebocytes and the transcription factor GLI1 is activated in sebocyte progenitors, suggesting a paracrine signaling mechanism. PTCH1 and IHH are up-regulated during human sebocyte differentiation in vitro and inhibition of hedgehog signaling inhibits growth and stimulates differentiation. Overexpression of DeltaNLef1 up-regulates IHH and stimulates proliferation of undifferentiated sebocytes. We present a model of the interactions between beta-catenin and hedgehog signaling in the epidermis in which SHH promotes proliferation of progenitors of the hair lineages whereas IHH stimulates proliferation of sebocyte precursors.
Figures
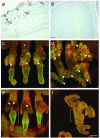
Fig. 1.
Expression of proteins in the PTCH-SHH signaling pathway in K14ΔNLef1 transgenic mouse sebaceous tumors. Immunostaining was performed with antibodies to the proteins indicated. (a) Ptch1 C terminus. (b) Ptch1 N terminus. (c) SHH. (d) IHH. (e) DHH. (f) pan-HH. (g) GLI1 (h) GLI2. (Scale bar: 200 μm.)
Fig. 2.
Expression of proteins in the SHH-PTCH signaling pathway in human sebaceous tumors. Immunostaining was performed with antibodies to the proteins indicated. (a) PTCH1 C terminus. (b) PTCH1 N terminus. (c) SHH. (d) IHH. (e) DHH. (f) pan-HH. (g) GLI1. (h) GLI2. (Scale bar: 200 μm.)
Fig. 3.
Expression of IHH, Gli1, and Gli2 proteins in normal and K14ΔNLef1 transgenic mouse skin and in human pilomatricoma. (a) Immunostaining with IHH antibody in normal mouse skin. Note strong reactivity in the sebaceous glands (arrowheads). (b) In human pilomatricoma (a hair follicle-derived tumor) immunostaining for IHH was not detected. (_c_-f) Immunostaining for GLI1 (green, c and d) and GLI2 (green, e and f) proteins in whole mounts of tail epidermis of WT (c and e) and K14ΔNLef1 transgenic (d and f) mice. Note strong expression of GLI1 in the nuclei of cells in the normal sebaceous glands (arrowheads in c) and newly differentiating sebocytes along the deformed transgenic hair follicles (arrowheads in d). Note strong and predominantly cytoplasmic staining of GLI2 in the permanent portion of the follicle below the sebaceous glands in WT epidermis (e, brackets) and weaker, more uniform staining in transgenic epidermis (f, bracket indicates equivalent region to those shown in e). Note weak nuclear staining for GLI2 in normal sebaceous glands (e, arrowheads). Whole mounts were double-labeled for β1 integrins (red) to reveal the basal IFE, periphery of sebaceous glands and outer root sheath of the hair follicles. [Scale bars: 100 μm (a and b) and 50 μm (_c_-f).]
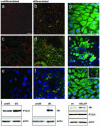
Fig. 4.
Expression of IHH, PTCH1, GLI1, and GLI2 in undifferentiated and differentiated human SZ95 sebocytes in vitro. (a, c, and e) Undifferentiated cells. (b, d, and f) Postconfluent, differentiated cells. (_g_-i) Partially differentiated cultures. (a and b) Nile red staining of lipids. (b) Note dramatic increase in the number of lipid droplets in differentiated SZ95 sebocytes (arrows). (c and d) Immunostaining for IHH protein (green) and E-cadherin (to reveal cell-cell borders; red). (d) IHH protein levels are up-regulated in differentiated sebocytes (arrows). (e and f) Immunostaining for PTCH1 protein (green) with 4′,6-diamidino-2-phenylindole nuclear counterstain (blue). PTCH1 staining is up-regulated in the cytoplasm of differentiated sebocytes (arrows in f). Immunostaining for GLI1 (g and h) and GLI2 (i) (green) with 4′,6-diamidino-2-phenylindole nuclear counterstain (blue), showing nuclear and cytoplasmic GLI localization in areas where the cultures are still undifferentiated (g and i) and exclusively cytoplasmic localization in suprabasal, terminally differentiating sebocytes (h and i Inset, arrows). [Scale bar: 20 μm(_a_-i).] (j) Western blots of protein lysates prepared from undifferentiated and differentiated SZ95 sebocytes (Left and Center) and retrovirally infected differentiated cells (Right) probed with antibodies against PTCH1 C terminus (160 kDa), pan-Hh (processed form of ≈25 kDa) and, as a loading control, actin (42 kDa). ev, empty vector control; ΔNLef1, retroviral vector.
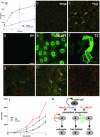
Fig. 5.
Manipulating Hh and β-catenin signaling regulates growth and differentiation of human sebocytes in vitro.(_a_-c) Inhibition of Hh signaling in human sebocytes decreases proliferation and stimulates differentiation. (a) Growth curve of SZ95 cells treated with Hh inhibitor cyclopamine or solvent methanol. (b) Treatment of undifferentiated SZ95 cells with methanol did not affect differentiation as evaluated by Nile red staining, whereas treatment with cyclopamine (c) resulted in accumulation of Nile red-positive lipid droplets (arrows). (_d_-j) Retroviral transduction of SZ95 cells with pBabepuro (d and g), pBabeΔNLef1 (e and h), and ΔNβ-catenin/T2 (f and i). Transduced gene products were detected by immunostaining with anti-Myc tag (e) and antihemagglutinin tag (d and f). Cells were labeled with antibodies specific for E-cadherin (to reveal cell-cell borders; red, _g_-i) and IHH (green, _g_-i). ΔNLef1 increased production of IHH protein (arrows in h). [Scale bars: 20 μm (_b_-i).] (j) Growth curve of cells transduced with pBabepuro (ev), pBabeΔNLef1 (dnLef1), or ΔNβ-catenin (T2). (k) Model of interactions between β-catenin and Hh signaling in epidermis. SHH promotes proliferation of progenitor cells of the hair lineage, whereas IHH stimulates proliferation of sebocyte precursors. The IHH signal is proposed to be produced by differentiated sebocytes and act on the sebocyte progenitors in a paracrine fashion.
Similar articles
- Beta-catenin and Hedgehog signal strength can specify number and location of hair follicles in adult epidermis without recruitment of bulge stem cells.
Silva-Vargas V, Lo Celso C, Giangreco A, Ofstad T, Prowse DM, Braun KM, Watt FM. Silva-Vargas V, et al. Dev Cell. 2005 Jul;9(1):121-31. doi: 10.1016/j.devcel.2005.04.013. Dev Cell. 2005. PMID: 15992546 - Characterization of bipotential epidermal progenitors derived from human sebaceous gland: contrasting roles of c-Myc and beta-catenin.
Lo Celso C, Berta MA, Braun KM, Frye M, Lyle S, Zouboulis CC, Watt FM. Lo Celso C, et al. Stem Cells. 2008 May;26(5):1241-52. doi: 10.1634/stemcells.2007-0651. Epub 2008 Feb 28. Stem Cells. 2008. PMID: 18308950 - Monstrous attempts at adnexogenesis: regulating hair follicle progenitors through Sonic hedgehog signaling.
Callahan CA, Oro AE. Callahan CA, et al. Curr Opin Genet Dev. 2001 Oct;11(5):541-6. doi: 10.1016/s0959-437x(00)00230-6. Curr Opin Genet Dev. 2001. PMID: 11532396 Review. - Clinical aspects of disrupted Hedgehog signaling (Review).
Oldak M, Grzela T, Lazarczyk M, Malejczyk J, Skopinski P. Oldak M, et al. Int J Mol Med. 2001 Oct;8(4):445-52. Int J Mol Med. 2001. PMID: 11562786 Review.
Cited by
- Wnt signaling and orthopedic diseases.
Ishikawa Y. Ishikawa Y. Am J Pathol. 2005 Jul;167(1):1-3. doi: 10.1016/S0002-9440(10)62947-1. Am J Pathol. 2005. PMID: 15972946 Free PMC article. Review. No abstract available. - Hedgehog signaling and gastrointestinal cancer.
Saqui-Salces M, Merchant JL. Saqui-Salces M, et al. Biochim Biophys Acta. 2010 Jul;1803(7):786-95. doi: 10.1016/j.bbamcr.2010.03.008. Epub 2010 Mar 19. Biochim Biophys Acta. 2010. PMID: 20307590 Free PMC article. Review. - Sebaceous gland organoid engineering.
Liu Y, Gao H, Chen H, Ji S, Wu L, Zhang H, Wang Y, Fu X, Sun X. Liu Y, et al. Burns Trauma. 2024 May 1;12:tkae003. doi: 10.1093/burnst/tkae003. eCollection 2024. Burns Trauma. 2024. PMID: 38699464 Free PMC article. Review. - Hedgehog Signaling Pathway Regulates the Proliferation and Differentiation of Rat Meibomian Gland Epithelial Cells.
Qu JY, Xiao YT, Zhang YY, Xie HT, Zhang MC. Qu JY, et al. Invest Ophthalmol Vis Sci. 2021 Feb 1;62(2):33. doi: 10.1167/iovs.62.2.33. Invest Ophthalmol Vis Sci. 2021. PMID: 33616621 Free PMC article. - Aging in the sebaceous gland.
Hou X, Wei Z, Zouboulis CC, Ju Q. Hou X, et al. Front Cell Dev Biol. 2022 Aug 17;10:909694. doi: 10.3389/fcell.2022.909694. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36060807 Free PMC article. Review.
References
- Niemann, C. & Watt, F. M. (2002) Trends Cell Biol. 12, 185-192. - PubMed
- Fuchs, E. & Raghavan, S. (2002) Nat. Rev. Genet. 3, 199-209. - PubMed
- Millar, S. E. (2002) J. Invest. Dermatol. 118, 216-225. - PubMed
- Owens, D. M. & Watt, F. M. (2003) Nat. Rev. Cancer 3, 444-451. - PubMed
- DasGupta, R. & Fuchs, E. (1999) Development (Cambridge, U.K.) 126, 4557-4568. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous