B7x: a widely expressed B7 family member that inhibits T cell activation - PubMed (original) (raw)
B7x: a widely expressed B7 family member that inhibits T cell activation
Xingxing Zang et al. Proc Natl Acad Sci U S A. 2003.
Abstract
B7 family proteins provide costimulatory signals that regulate T cell responses. Here we report the third set of B7 family-related T cell inhibitory molecules with the identification of a homolog of the B7 family, B7x. It is expressed in immune cells, nonlymphoid tissues, and some tumor cell lines. B7x inhibits cell-cycle progression, proliferation, and cytokine production of both CD4+ and CD8+ T cells. B7x binds a receptor that is expressed on activated, but not resting T cells that is distinct from known CD28 family members. Its receptor may be a recently identified inhibitory molecule, B and T lymphocyte attenuator. These studies identify a costimulatory pathway that may have a unique function in downregulation of tissue-specific autoimmunity and antitumor responses.
Figures
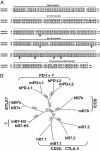
Fig. 1.
B7x is a member of the B7 family. (A) Comparison of human B7x with mouse B7x. Predicted signal peptide, Ig V-like and C-like domains, and the hydrophobic/transmembrane region are indicated. Identical amino acids are highlighted in black, and similar residues are shaded in gray. The potential N-glycosylation sites are indicated by arrows. (B) Phylogenetic tree of mouse and human B7 family generated by
paup* version 4.0b10
. Numbers show the percentage of bootstrap support for each clade.
Fig. 2.
B7x is widely expressed in multiple tissues and tumor lines. (A) Real-time PCR was performed on cDNA from multiple mouse tissues. cDNA from the CLONTECH mouse multiple-tissue cDNA panel I was used as well as cDNA made from tissues dissected out of two C57/BL6 mice. The error bars represent the SD among the different mouse cDNA samples. (B) Real-time PCR was performed on CD11c+ dendritic cells, B cells, and T cells that were purified from the spleen and compared to the whole spleen. Thioglycolate-induced macrophages were purified by overnight adherence and removal of nonadherent cells. The results shown are the average and SD among 4-10 individual mouse samples. (C) RT-PCR analysis of B7x expression in mouse tumor cell lines.
Fig. 3.
B7x inhibits T cell activation. (A) Purified T cells and CD4+/CD8+ subsets from BALB/c mice were stimulated with plate-bound anti-CD3 (0.25 μg/ml for CD4+ and total T cells, 2 μg/ml for CD8+ T cells) and plate-bound B7x-Ig (▾) or control Ig (•). Aliquots of supernatants were collected 48 h after initiation of cultures, cytokines were measured by ELISA, and proliferation was measured after 72 h by thymidine incorporation. Error bars indicate SD of triplicate cultures. These data are representative of three independent experiments. (B) Purified T cells were stimulated with plate-bound anti-CD3 and CHO transfectants expressing GFP (•), B7-2 (○), or B7x (▾). Cytokines and proliferation were measured as above. These data are representative of five independent experiments. (C) T cells were labeled with CFSE and stimulated with or without plate-bound anti-CD3 and CHO transfectants expressing GFP or B7x. On day 4, cells were harvested, stained with PE-anti-CD4 or PE-anti-CD8, and analyzed by flow cytometry. Percentages refer to fraction of cells in the nondividing peak or cells that have divided more than twice. These data are representative of three independent experiments.
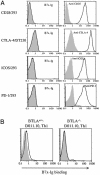
Fig. 4.
B7x has a putative counterreceptor on activated T cells distinct from CD28, CTLA-4, ICOS, and PD-1. (A) The 293 transfectants expressing CD28, ICOS, and PD-l or the DT230 transfectant expressing cell surface CTLA-4 were stained with B7x-Ig fusion protein (open histograms) or mouse IgG1 (shaded histograms) as a control, and were then stained with a PE-conjugate anti-mouse-IgG. All transfectants were also stained with specific mAbs (open histograms) or control Abs (shaded histograms). (B) DO11.10 T cell antigen receptor transgenic Th1 cells from WT or BTLA-/- mice were incubated with irradiated BALB/c spleen cells in the presence of 5 μg/ml OVA323-339 peptide for 4 days. Cells were then incubated with B7x-Ig (open histograms) or human IgG1 (shaded histograms) as a control and stained with a PE-conjugated anti-human-IgG.
Similar articles
- Dynamic equilibrium of B7-1 dimers and monomers differentially affects immunological synapse formation and T cell activation in response to TCR/CD28 stimulation.
Bhatia S, Sun K, Almo SC, Nathenson SG, Hodes RJ. Bhatia S, et al. J Immunol. 2010 Feb 15;184(4):1821-8. doi: 10.4049/jimmunol.0902869. Epub 2010 Jan 11. J Immunol. 2010. PMID: 20065109 Free PMC article. - B7-H1 expression on old CD8+ T cells negatively regulates the activation of immune responses in aged animals.
Mirza N, Duque MA, Dominguez AL, Schrum AG, Dong H, Lustgarten J. Mirza N, et al. J Immunol. 2010 May 15;184(10):5466-5474. doi: 10.4049/jimmunol.0903561. Epub 2010 Apr 7. J Immunol. 2010. PMID: 20375308 Free PMC article. - Differential down-regulation of CD28 by B7-1 and B7-2 engagement.
Eck SC, Chang D, Wells AD, Turka LA. Eck SC, et al. Transplantation. 1997 Nov 27;64(10):1497-9. doi: 10.1097/00007890-199711270-00025. Transplantation. 1997. PMID: 9392322 - The right place at the right time: novel B7 family members regulate effector T cell responses.
Liang L, Sha WC. Liang L, et al. Curr Opin Immunol. 2002 Jun;14(3):384-90. doi: 10.1016/s0952-7915(02)00342-4. Curr Opin Immunol. 2002. PMID: 11973139 Review. - T cell costimulatory and inhibitory receptors as therapeutic targets for inducing anti-tumor immunity.
Foell J, Hewes B, Mittler RS. Foell J, et al. Curr Cancer Drug Targets. 2007 Feb;7(1):55-70. doi: 10.2174/156800907780006841. Curr Cancer Drug Targets. 2007. PMID: 17305478 Review.
Cited by
- Glycosylation and Its Role in Immune Checkpoint Proteins: From Molecular Mechanisms to Clinical Implications.
Liu J, Xu X, Zhong H, Yu M, Abuduaini N, Zhang S, Yang X, Feng B. Liu J, et al. Biomedicines. 2024 Jun 28;12(7):1446. doi: 10.3390/biomedicines12071446. Biomedicines. 2024. PMID: 39062019 Free PMC article. Review. - Clinical Perspectives to Overcome Acquired Resistance to Anti-Programmed Death-1 and Anti-Programmed Death Ligand-1 Therapy in Non-Small Cell Lung Cancer.
Lee YJ, Lee JB, Ha SJ, Kim HR. Lee YJ, et al. Mol Cells. 2021 May 31;44(5):363-373. doi: 10.14348/molcells.2021.0044. Mol Cells. 2021. PMID: 34001680 Free PMC article. Review. - Not All Immune Checkpoints Are Created Equal.
De Sousa Linhares A, Leitner J, Grabmeier-Pfistershammer K, Steinberger P. De Sousa Linhares A, et al. Front Immunol. 2018 Aug 31;9:1909. doi: 10.3389/fimmu.2018.01909. eCollection 2018. Front Immunol. 2018. PMID: 30233564 Free PMC article. Review. - A CD300c-Fc Fusion Protein Inhibits T Cell Immunity.
Cui C, Su M, Lin Y, Lai L. Cui C, et al. Front Immunol. 2018 Nov 15;9:2657. doi: 10.3389/fimmu.2018.02657. eCollection 2018. Front Immunol. 2018. PMID: 30498497 Free PMC article. - B7-H4 mediates inhibition of T cell responses by activated murine hepatic stellate cells.
Chinnadurai R, Grakoui A. Chinnadurai R, et al. Hepatology. 2010 Dec;52(6):2177-85. doi: 10.1002/hep.23953. Epub 2010 Nov 9. Hepatology. 2010. PMID: 21064155 Free PMC article.
References
- Egen, J. G., Kuhns, M. S. & Allison, J. P. (2002) Nat. Immunol. 3, 611-618. - PubMed
- Sharpe, A. H. & Freeman, G. J. (2002) Nat. Rev. Immunol. 2, 116-126. - PubMed
- Abbas, A. K. & Sharpe, A. H. (1999) Nat. Med. 5, 1345-1346. - PubMed
- Coyle, A. J. & Gutierrez-Ramos, J. C. (2001) Nat. Immunol. 2, 203-209. - PubMed
- Carreno, B. M. & Collins, M. (2002) Annu. Rev. Immunol. 20, 29-53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials