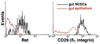Hirschsprung disease is linked to defects in neural crest stem cell function - PubMed (original) (raw)
Hirschsprung disease is linked to defects in neural crest stem cell function
Toshihide Iwashita et al. Science. 2003.
Abstract
Genes associated with Hirschsprung disease, a failure to form enteric ganglia in the hindgut, were highly up-regulated in gut neural crest stem cells relative to whole-fetus RNA. One of these genes, the glial cell line-derived neurotrophic factor (GDNF) receptor Ret, was necessary for neural crest stem cell migration in the gut. GDNF promoted the migration of neural crest stem cells in culture but did not affect their survival or proliferation. Gene expression profiling, combined with reverse genetics and analyses of stem cell function, suggests that Hirschsprung disease is caused by defects in neural crest stem cell function.
Figures
Fig. 1
Flow-cytometric analysis of Ret, and CD29 (β1 integrin) expression by E14.5 gut p75+α4+ NCSCs and E14.5 gut p75−α4− epithelial progenitors from the same dissociated guts. As summarized in Table 2, the gut NCSCs consistently expressed Ret and CD29. In contrast, gut epithelial progenitors did not detectably express Ret but heterogeneously expressed CD29.
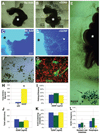
Fig. 2
GDNF signaling promotes gut NCSC migration and is required for the migration of NCSCs into the intestines. [(A) to(E)] In nine independent experiments, E13.5 toE14.5 rat guts (*) were dissected and cultured in collagen gels. In the absence of GDNF (A and C), few cells migrated out of the gut, whereas in the presence of GDNF (10 ng/ml) (B and D), a large number of cells migrated into the collagen gel [(A) and (B): tiled phase-contrast images; scale bars, 400 µm; (C) and (D): Hoechst 33342–stained nuclei; scale bar, 200 µm]. In GDNF-supplemented cultures, many cells migrated along neurites that extended into the collagen [(D), arrowhead]. (E) Neural crest cells migrated in the direction of beads (arrow) soaked in GDNF. Scale bar, 400 µm. (F and G) Migrating cells that were extracted from the gel and cultured at clonal density formed large multilineage NCSC colonies containing neurons [peripherin+, shown in (F)], glia [GFAP+, shown in (G)], and myofibroblasts [SMA+, shown in (G)]. Scale bar in (F) and (G), 50 µm. (H) In three independent experiments, 13 times as many (*P < 0.001) NCSCs were extracted and cultured from GDNF-supplemented gels. In five to seven independent experiments, GDNF did not affect the ability of single E14.5 gut NCSCs to survive (**I**) or proliferate over the first 6 days in culture (**J**). (**K**) In four independent experiments, GDNF also did not affect the percentage of p75+α4+ NCSCs that differentiated to form colonies containing neurons and glia in culture. (**L**) The frequency of NCSCs that could be cultured from _Ret_−/− esophagus was reduced by a factor of 4 (_P_ = 0.07), but in three independent experiments, _Ret_−/− NCSCs were nearly absent from the stomach and intestines (factor of >20 reduction; *P < 0.05). Similar results were obtained in two experiments using E15.5 guts. GDNF also did not affect E12.5 or E14.5 NCSC survival, or proliferation in chemically defined standard medium lacking chick embryo extract (fig. S5).
Comment in
- Stem cell defects as a possible cause of Hirschsprung's disease.
Hasler WL. Hasler WL. Gastroenterology. 2004 Apr;126(4):1205-7; discussion 1207. doi: 10.1053/j.gastro.2003.11.064. Gastroenterology. 2004. PMID: 15057763 No abstract available.
Similar articles
- Signalling by the RET receptor tyrosine kinase and its role in the development of the mammalian enteric nervous system.
Taraviras S, Marcos-Gutierrez CV, Durbec P, Jani H, Grigoriou M, Sukumaran M, Wang LC, Hynes M, Raisman G, Pachnis V. Taraviras S, et al. Development. 1999 Jun;126(12):2785-97. doi: 10.1242/dev.126.12.2785. Development. 1999. PMID: 10331988 - Neural cells in the esophagus respond to glial cell line-derived neurotrophic factor and neurturin, and are RET-dependent.
Yan H, Bergner AJ, Enomoto H, Milbrandt J, Newgreen DF, Young HM. Yan H, et al. Dev Biol. 2004 Aug 1;272(1):118-33. doi: 10.1016/j.ydbio.2004.04.025. Dev Biol. 2004. PMID: 15242795 - Requirement of signalling by receptor tyrosine kinase RET for the directed migration of enteric nervous system progenitor cells during mammalian embryogenesis.
Natarajan D, Marcos-Gutierrez C, Pachnis V, de Graaff E. Natarajan D, et al. Development. 2002 Nov;129(22):5151-60. doi: 10.1242/dev.129.22.5151. Development. 2002. PMID: 12399307 - The GDNF-RET signalling partnership.
Robertson K, Mason I. Robertson K, et al. Trends Genet. 1997 Jan;13(1):1-3. doi: 10.1016/s0168-9525(96)30113-3. Trends Genet. 1997. PMID: 9009838 Review. No abstract available. - [Molecular basis of Hirschsprung disease].
Inoue M, Okada A. Inoue M, et al. Nihon Rinsho. 1998 Jan;56(1):249-57. Nihon Rinsho. 1998. PMID: 9465697 Review. Japanese.
Cited by
- Direct isolation of myofibroblasts and fibroblasts from bleomycin-injured lungs reveals their functional similarities and differences.
Akamatsu T, Arai Y, Kosugi I, Kawasaki H, Meguro S, Sakao M, Shibata K, Suda T, Chida K, Iwashita T. Akamatsu T, et al. Fibrogenesis Tissue Repair. 2013 Aug 8;6(1):15. doi: 10.1186/1755-1536-6-15. Fibrogenesis Tissue Repair. 2013. PMID: 23927729 Free PMC article. - Chronic Megacolon Presenting in Adolescents or Adults: Clinical Manifestations, Diagnosis, and Genetic Associations.
Wang XJ, Camilleri M. Wang XJ, et al. Dig Dis Sci. 2019 Oct;64(10):2750-2756. doi: 10.1007/s10620-019-05605-7. Epub 2019 Apr 5. Dig Dis Sci. 2019. PMID: 30953226 Free PMC article. Review. - Class 3 semaphorins negatively regulate dermal lymphatic network formation.
Uchida Y, James JM, Suto F, Mukouyama YS. Uchida Y, et al. Biol Open. 2015 Aug 28;4(9):1194-205. doi: 10.1242/bio.012302. Biol Open. 2015. PMID: 26319580 Free PMC article. - Central role of the threonine residue within the p+1 loop of receptor tyrosine kinase in STAT3 constitutive phosphorylation in metastatic cancer cells.
Yuan ZL, Guan YJ, Wang L, Wei W, Kane AB, Chin YE. Yuan ZL, et al. Mol Cell Biol. 2004 Nov;24(21):9390-400. doi: 10.1128/MCB.24.21.9390-9400.2004. Mol Cell Biol. 2004. PMID: 15485908 Free PMC article. - Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells.
Shimono Y, Zabala M, Cho RW, Lobo N, Dalerba P, Qian D, Diehn M, Liu H, Panula SP, Chiao E, Dirbas FM, Somlo G, Pera RA, Lao K, Clarke MF. Shimono Y, et al. Cell. 2009 Aug 7;138(3):592-603. doi: 10.1016/j.cell.2009.07.011. Cell. 2009. PMID: 19665978 Free PMC article.
References
- Weissman IL. Science. 2000;287:1442. - PubMed
- Phillips RL, et al. Science. 2000;288:1635. - PubMed
- Geschwind DH, et al. Neuron. 2001;29:325. - PubMed
- Park IK, et al. Blood. 2002;99:488. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS040750/NS/NINDS NIH HHS/United States
- R21 HD40760-02/HD/NICHD NIH HHS/United States
- NIH5P60-DK20572/DK/NIDDK NIH HHS/United States
- P60-AR20557/AR/NIAMS NIH HHS/United States
- P30 AR48310/AR/NIAMS NIH HHS/United States
- CA46592/CA/NCI NIH HHS/United States
- R01 NS040750-01/NS/NINDS NIH HHS/United States
- R01 NS40750-01/NS/NINDS NIH HHS/United States
- DK58771/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources