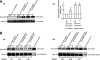ARF6 regulates a plasma membrane pool of phosphatidylinositol(4,5)bisphosphate required for regulated exocytosis - PubMed (original) (raw)
ARF6 regulates a plasma membrane pool of phosphatidylinositol(4,5)bisphosphate required for regulated exocytosis
Yoshikatsu Aikawa et al. J Cell Biol. 2003.
Abstract
ADP-ribosylation factor (ARF) 6 regulates endosomal plasma membrane trafficking in many cell types, but is also suggested to play a role in Ca2+-dependent dense-core vesicle (DCV) exocytosis in neuroendocrine cells. In the present work, expression of the constitutively active GTPase-defective ARF6Q67L mutant in PC12 cells was found to inhibit Ca2+-dependent DCV exocytosis. The inhibition of exocytosis was accompanied by accumulation of ARFQ67L, phosphatidylinositol 4,5-bisphosphate (PIP2), and the phosphatidylinositol 4-phosphate 5-kinase type I (PIP5KI) on endosomal membranes with their corresponding depletion from the plasma membrane. That the depletion of PIP2 and PIP5K from the plasma membrane caused the inhibition of DCV exocytosis was demonstrated directly in permeable cell reconstitution studies in which overexpression or addition of PIP5KIgamma restored Ca2+-dependent exocytosis. The restoration of exocytosis in ARF6Q67L-expressing permeable cells unexpectedly exhibited a Ca2+ dependence, which was attributed to the dephosphorylation and activation of PIP5K. Increased Ca2+ and dephosphorylation stimulated the association of PIP5KIgamma with ARF6. The results reveal a mechanism by which Ca2+ influx promotes increased ARF6-dependent synthesis of PIP2. We conclude that ARF6 plays a role in Ca2+-dependent DCV exocytosis by regulating the activity of PIP5K for the synthesis of an essential plasma membrane pool of PIP2.
Figures
Figure 1.
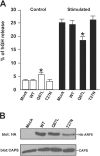
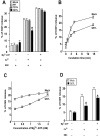
ARF6Q67L localizes to plasma membrane and endosomes in PC12 cells and inhibits Ca2 + -dependent DCV exocytosis. PC12 cells were transfected with pcDNA3 (mock), pXS-ARF6WT, ARF6Q67L, and ARF6T27N plasmids along with plasmid encoding hGH. (A) 48 h after transfection, cells were incubated for 20 min with calcium-free Locke's solution (control) or a depolarizing concentration of K+ (stimulated), and hGH release was determined (mean values ± SEM, n = 9). Data are respresentative of three independent experiments, and statistically significant (P < 0.005) differences are marked by asterisks. (B) Western blots showing similar expression levels for HA-ARF6WT and HA-ARF6Q67L proteins and a lower level for the HA-ARF6T27N protein. CAPS was detected as a loading control. (C) Intracellular distribution of hGH and chromogranin B (CgB) in transfected PC12 cells. Cells expressing indicated HA-tagged ARF6 proteins and hGH were analyzed by indirect immunofluorescence with HA (for ARF6, red) and hGH (green) antibodies or with ARF6 (red) and CgB (green) antibodies. Arrowheads indicate docked hGH- or CgB-containing DCVs that lack ARF6, and arrows indicate ARF6-positive cytoplasmic vesicles that lack hGH and CgB. Insets show magnified boxed regions. Bar, 5 μm.
Figure 1.
ARF6Q67L localizes to plasma membrane and endosomes in PC12 cells and inhibits Ca2 + -dependent DCV exocytosis. PC12 cells were transfected with pcDNA3 (mock), pXS-ARF6WT, ARF6Q67L, and ARF6T27N plasmids along with plasmid encoding hGH. (A) 48 h after transfection, cells were incubated for 20 min with calcium-free Locke's solution (control) or a depolarizing concentration of K+ (stimulated), and hGH release was determined (mean values ± SEM, n = 9). Data are respresentative of three independent experiments, and statistically significant (P < 0.005) differences are marked by asterisks. (B) Western blots showing similar expression levels for HA-ARF6WT and HA-ARF6Q67L proteins and a lower level for the HA-ARF6T27N protein. CAPS was detected as a loading control. (C) Intracellular distribution of hGH and chromogranin B (CgB) in transfected PC12 cells. Cells expressing indicated HA-tagged ARF6 proteins and hGH were analyzed by indirect immunofluorescence with HA (for ARF6, red) and hGH (green) antibodies or with ARF6 (red) and CgB (green) antibodies. Arrowheads indicate docked hGH- or CgB-containing DCVs that lack ARF6, and arrows indicate ARF6-positive cytoplasmic vesicles that lack hGH and CgB. Insets show magnified boxed regions. Bar, 5 μm.
Figure 2.
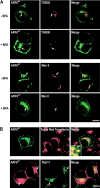
ARF6 localizes to the endocytic pathway in PC12 cells. (A) PC12 cells were transfected for 48 h with wild-type EGFP-ARF6 (green) and treated with 5 μg ml−1 BFA where indicated (BFA). Immunocytochemistry was conducted to localize TGN38 (red) and mannosidase II (red). Arrows indicate ARF6-containing compartments that lack TGN38 and mannosidase II. (B) Top row, internalized Texas red transferrin (red) exhibits extensive colocalization with EGFP-ARF6 (green). Inset shows magnified perinuclear region. Bottom row, HA-ARF6 (red) exhibits extensive colocalization with Rab11 (green). Arrows indicate ARF6-containing compartments that colocalize with transferrin and Rab11. Bars, 10 μm.
Figure 3.
PIP2 redistributes from plasma membrane to endosomes in ARF6Q67L-expressing PC12 cells. (A) PC12 cells were cotransfected for 48 h with plasmids encoding PH-GFP (green) and HA-tagged ARF6WT, ARF6Q67L, or ARF6T27N (detected with HA antibody, red). Insets show enlarged regions of the plasma membrane. Cells expressing relatively high levels of ARF6Q67L exhibited endosomal accumulation of PH-GFP (arrow) and decreased plasma membrane fluorescence (inset). (B) The fluorescence intensity of PH-GFP was quantified in 50 randomly selected cells, and the ratio of plasma membrane (left) or endosomal (right) to total fluorescence was plotted as mean values ± SEM (asterisk indicates P < 0.005 for comparison with mock-transfected by t test). (C) Immunoreactive PIP2 (red) was detected in permeable PC12 cells expressing ARF6WT, ARFQ67L, or ARF6T27N (green). Immunoreactive PIP2 was decreased in ARF6Q67L-expressing cells (arrows). (D) The fluorescence intensity of PIP2 antibody staining was quantified and normalized to membrane area in 50 randomly selected cells. Values are means ± SEM with asterisk corresponding to P < 0.005 for comparison with mock-transfected by t test). Bar, 20 μm.
Figure 3.
PIP2 redistributes from plasma membrane to endosomes in ARF6Q67L-expressing PC12 cells. (A) PC12 cells were cotransfected for 48 h with plasmids encoding PH-GFP (green) and HA-tagged ARF6WT, ARF6Q67L, or ARF6T27N (detected with HA antibody, red). Insets show enlarged regions of the plasma membrane. Cells expressing relatively high levels of ARF6Q67L exhibited endosomal accumulation of PH-GFP (arrow) and decreased plasma membrane fluorescence (inset). (B) The fluorescence intensity of PH-GFP was quantified in 50 randomly selected cells, and the ratio of plasma membrane (left) or endosomal (right) to total fluorescence was plotted as mean values ± SEM (asterisk indicates P < 0.005 for comparison with mock-transfected by t test). (C) Immunoreactive PIP2 (red) was detected in permeable PC12 cells expressing ARF6WT, ARFQ67L, or ARF6T27N (green). Immunoreactive PIP2 was decreased in ARF6Q67L-expressing cells (arrows). (D) The fluorescence intensity of PIP2 antibody staining was quantified and normalized to membrane area in 50 randomly selected cells. Values are means ± SEM with asterisk corresponding to P < 0.005 for comparison with mock-transfected by t test). Bar, 20 μm.
Figure 4.
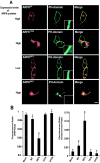
PIP5KIγ is recruited to an endosomal compartment in ARF6Q67L-expressing PC12 cells. PC12 cells were cotransfected for 48 h with plasmids encoding EGFP-ARF6 proteins (green) and HA-PIP5KIγ (detected by HA antibody, red). Insets show magnified regions of the plasma membrane. Cells expressing relatively high levels of ARF6Q67L exhibited an endosomal accumulation of PIP5KIγ (arrow) and its depletion from the plasma membrane (inset). Bar, 10 μm.
Figure 5.
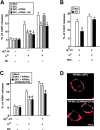
The inhibition of DCV exocytosis by ARF6Q67L is preserved in permeable cells and reversed in incubations containing Ca2 + , MgATP, and cytosol. PC12 cells were cotransfected for 48 h with plasmids encoding hGH and ARF6WT, ARF6T27N, or ARF6Q67L as indicated, and permeable cell hGH secretion assays were conducted (shown as mean ± SEM, n = 4). (A) Inhibition of DCV exocytosis in ARF6Q67L-expressing cells was preserved on permeabilization, but only in incubations lacking MgATP. Incubations contained Ca2+, MgATP, and rat brain cytosol (RBC) as indicated. (B) Reversal of inhibition in ARF6Q67L-expressing cells required all three components. Two-stage secretion assays were conducted with MgATP and cytosol in priming incubations and Ca2+ and cytosol in triggering incubations (for times indicated). (C) Reversal of inhibition in ARF6Q67L-expressing cells required MgATP. Incubations similar to those in A were conducted with Ca2+, cytosol, and indicated concentrations of MgATP. (D) Reversal of inhibition in ARF6Q67L-expressing cells was not observed in the absence of cytosol. Incubations similar to those in A were conducted with Ca2+ or MgATP in the absence of cytosol (RBC). Asterisks indicate significant (P < 0.01) differences with mock- transfected cells.
Figure 6.
Expression or addition of PIPKIγ to permeable cells replaces cytosol in restoring DCV exocytosis in ARF6 Q67 L-expressing cells. PC12 cells were transfected for 48 h with plasmids encoding hGH and either ARF6Q67Lor PIP5KIγ as indicated, and permeable cell hGH secretion assays were conducted. (A) Expression of PIP5KIγ restored Ca2+-dependent hGH secretion in ARF6Q67L- expressing cells in the presence (but not in the absence) of MgATP. Expression of the Δ345 PIP5KIγ mutant failed to restore Ca2+-dependent secretion. (B) Purified PIP5KIγ replaced cytosol and restored Ca2+-dependent hGH secretion in ARF6Q67L- expressing cells. Incubations similar to those of A were conducted with Ca2+, MgATP, and 0.6 μg/ml (∼7 nM) PIP5KIγ where indicated. (C) Expression of PIP5KIα, but not a kinase-dead (D309N/R427Q) mutant, replaced cytosol in restoring Ca2+-dependent hGH secretion in ARF6Q67L-expressing cells in the presence (but not in the absence) of MgATP. Results are representative of at least two experiments with mean hGH values ± SEM (n = 4). (D) Wild-type and kinase-dead FLAG-tagged PIP5KIα proteins were expressed at similar levels and were plasma membrane localized. Immunocytochemistry with FLAG antibodies was conducted. Bar, 10 μm. Asterisks indicate significant (P < 0.01) differences with mock-transfected cells.
Figure 7.
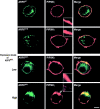
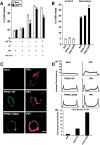
PIP5KIγ is regulated by Ca 2**+** and dephosphorylation. (A) The restoration of Ca2+-dependent hGH secretion in permeable ARF6Q67L- expressing cells by Ca2+, MgATP, and cytosol is prevented by inhibition of PKC. Permeable cell incubations were conducted 48 h after transfection with hGH- and ARF6Q67L-encoding plasmids. Restoration of Ca2+-dependent hGH release was observed with MgATP and cytosol, but not in the presence of 5 μg/ml PKC(19–31) pseudosubstrate inhibitor. (B) Expression of S264A PIP5KIγ (but not wild-type PIP5KIγ) enhanced Ca2+-dependent hGH secretion. PC12 cells were cotransfected with plasmids encoding hGH and wild-type or S264A PIP5KIγ as indicated. hGH release from intact cells in Na+ (control) or K+ (stimulated) buffers was determined. Asterisks indicate significant (P < 0.05) differences with mock-transfected cells. (C) PC12 cells were probed with HA antibody to detect PIP5KIγ (green) and PIP2 antibody to detect PIP2 (red). Plasma membrane immunoreactive PIP2 increased in cells expressing S264A PIP5KIγ. Bar, 10 μm. (D, a) Quantitation of PIP2 in cells expressing PIP5KIγ or S264A PIP5KIγ. A representative density profile plot was generated using SCION Image for green channel (PIP5K, left) and red channel (PIP2, right). Pixel intensity was determined by a “row average plot” of a section selected from a confocal slice through the center of the cell. (D, b) The percentage of cells exhibiting average pixel intensities above 150 for immunoreactive PIP2 is shown for mock transfectants and cells expressing wild-type or S264A PIP5KIγ. 50 randomly selected cells of each type were quantitated.
Figure 8.
ARF6 associates with PIP5KIγ. PC12 cells were cotransfected with plasmids encoding EGFP-ARF6WT, EGFP-ARF6Q67L, or EGFP-ARF6N122I and HA-tagged wild-type or S264A PIP5KIγ as indicated. (A, a) Ca2+ influx and dephosphorylation promote the association of ARF6 with PIP5KIγ. Cells cotransfected for 48 h with indicated plasmids were incubated in Na+-containing (control, CNT) or K+-containing (stimulated, S) buffers for 5 min. HA antibody immunoprecipitates were prepared from detergent lysates and analyzed by Western blotting for EGFP-ARF6 and HA-PIP5KIγ. Co-immunoprecipitation of EGFP-ARF6 with HA antibody was enhanced by K+ depolarization in PIP5KIγ-expressing cells. In S264A PIP5KIγ-expressing cells, coimmunoprecipitation was increased in resting cells and not enhanced by K+ depolarization. (A, b) Quantitation of ARF6 coimmunoprecipitation with PIP5KIγ from 3 experiments (± SEM) similar to that in panel a. (B) Co-immunoprecipitation studies similar to those in panel A were conducted for cells cotransfected with plasmids encoding ARF6WT, ARF6Q67L, and ARF6N122I with (a) wild-type HA-PIP5KIγ or (b) HA-S264A PIP5KIγ. Values under each lane pair indicate fold stimulation by K+ depolarization.
Figure 9.
ARF6 regulation of Ca 2**+** -dependent DCV exocytosis in PC12 cells. (1) Ca2+ influx stimulates exocytosis of docked DCVs, but only if plasma membrane PIP2 is available. (2) Ca2+ influx promotes the dephosphorylation of PIP5KI. (3) Dephospho-PIP5KI associates with ARF6 and is activated to synthesize PIP2 for DCV exocytosis. (4) Increased PIP2 synthesis drives endocytosis and retrieval of the DCV membrane after exocytosis. (5) Constitutive endocytosis may be enhanced by ARF6Q67L stimulation of PIP5K. (6 and 7) Lack of GTP hydrolysis on ARF6Q67L and constitutive PIP2 production on endosomes prevents recycling to the plasma membrane. Entrapment of plasma membrane constituents and diversion of PIP5K and PIP2 to endosomes in ARF6Q67L-expressing cells results in the inhibition of DCV exocytosis.
Similar articles
- ADP-ribosylation factor 6 regulation of phosphatidylinositol-4,5-bisphosphate synthesis, endocytosis, and exocytosis.
Aikawa Y, Martin TF. Aikawa Y, et al. Methods Enzymol. 2005;404:422-31. doi: 10.1016/S0076-6879(05)04037-1. Methods Enzymol. 2005. PMID: 16413288 - Phosphatidylinositol 4,5-bisphosphate and Arf6-regulated membrane traffic.
Brown FD, Rozelle AL, Yin HL, Balla T, Donaldson JG. Brown FD, et al. J Cell Biol. 2001 Sep 3;154(5):1007-17. doi: 10.1083/jcb.200103107. J Cell Biol. 2001. PMID: 11535619 Free PMC article. - Calcium-regulated exocytosis of dense-core vesicles requires the activation of ADP-ribosylation factor (ARF)6 by ARF nucleotide binding site opener at the plasma membrane.
Vitale N, Chasserot-Golaz S, Bailly Y, Morinaga N, Frohman MA, Bader MF. Vitale N, et al. J Cell Biol. 2002 Oct 14;159(1):79-89. doi: 10.1083/jcb.200203027. Epub 2002 Oct 14. J Cell Biol. 2002. PMID: 12379803 Free PMC article. - Regulation of PIP5K activity by Arf6 and its physiological significance.
Funakoshi Y, Hasegawa H, Kanaho Y. Funakoshi Y, et al. J Cell Physiol. 2011 Apr;226(4):888-95. doi: 10.1002/jcp.22482. J Cell Physiol. 2011. PMID: 20945365 Review. - Potential regulation of ADP-ribosylation factor 6 signalling by phosphatidylinositol 3,4,5-trisphosphate.
Cullen PJ, Venkateswarlu K. Cullen PJ, et al. Biochem Soc Trans. 1999 Aug;27(4):683-9. doi: 10.1042/bst0270683. Biochem Soc Trans. 1999. PMID: 10917667 Review.
Cited by
- Supervised membrane swimming: small G-protein lifeguards regulate PIPK signalling and monitor intracellular PtdIns(4,5)P2 pools.
Santarius M, Lee CH, Anderson RA. Santarius M, et al. Biochem J. 2006 Aug 15;398(1):1-13. doi: 10.1042/BJ20060565. Biochem J. 2006. PMID: 16856876 Free PMC article. Review. - A mechanism for exocyst-mediated tethering via Arf6 and PIP5K1C-driven phosphoinositide conversion.
Maib H, Murray DH. Maib H, et al. Curr Biol. 2022 Jul 11;32(13):2821-2833.e6. doi: 10.1016/j.cub.2022.04.089. Epub 2022 May 23. Curr Biol. 2022. PMID: 35609603 Free PMC article. - Localization of phosphatidylinositol 4-phosphate 5-kinase (PIP5K) α, β, γ in the three major salivary glands in situ of mice and their response to β-adrenoceptor stimulation.
Khrongyut S, Rawangwong A, Pidsaya A, Sakagami H, Kondo H, Hipkaeo W. Khrongyut S, et al. J Anat. 2019 Apr;234(4):502-514. doi: 10.1111/joa.12944. Epub 2019 Feb 7. J Anat. 2019. PMID: 30734271 Free PMC article. - Anx2 interacts with HIV-1 Gag at phosphatidylinositol (4,5) bisphosphate-containing lipid rafts and increases viral production in 293T cells.
Harrist AV, Ryzhova EV, Harvey T, González-Scarano F. Harrist AV, et al. PLoS One. 2009;4(3):e5020. doi: 10.1371/journal.pone.0005020. Epub 2009 Mar 27. PLoS One. 2009. PMID: 19325895 Free PMC article. - Resolution of structure of PIP5K1A reveals molecular mechanism for its regulation by dimerization and dishevelled.
Hu J, Yuan Q, Kang X, Qin Y, Li L, Ha Y, Wu D. Hu J, et al. Nat Commun. 2015 Sep 14;6:8205. doi: 10.1038/ncomms9205. Nat Commun. 2015. PMID: 26365782 Free PMC article.
References
- Anderson, R.A., I.V. Boronenkov, S.D. Doughman, J. Lunz, and J.C. Loijens. 1999. Phosphatidylinositol phosphate kinases, a multifaceted family of signaling enzymes. J. Biol. Chem. 274:9907–9910. - PubMed
- Banerjee, A., J.A. Kowalchyk, B.R. DasGupta, and T.F. Martin. 1996. SNAP-25 is required for a late postdocking step in Ca2+-dependent exocytosis. J. Biol. Chem. 271:20227–20230. - PubMed
- Barbieri, M.A., C.M. Heath, E.M. Peters, A. Wells, J.N. Davis, and P.D. Stahl. 2001. Phosphatidylinositol-4-phosphate 5-kinase-1beta is essential for epidermal growth factor receptor-mediated endocytosis. J. Biol. Chem. 276:47212–47216. - PubMed
- Barylko, B., S.H. Gerber, D.D. Binns, N. Grichine, M. Khvotchev, T.C. Sudhof, and J.P. Albanesi. 2001. A novel family of phosphatidylinositol 4-kinases conserved from yeast to humans. J. Biol. Chem. 276:7705–7708. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous