Mycobacterium's arrest of phagosome maturation in macrophages requires Rab5 activity and accessibility to iron - PubMed (original) (raw)
Mycobacterium's arrest of phagosome maturation in macrophages requires Rab5 activity and accessibility to iron
Victoria A Kelley et al. Mol Biol Cell. 2003 Aug.
Abstract
Many mycobacteria are intramacrophage pathogens that reside within nonacidified phagosomes that fuse with early endosomes but do not mature to phagolysosomes. The mechanism by which mycobacteria block this maturation process remains elusive. To gain insight into whether fusion with early endosomes is required for mycobacteria-mediated inhibition of phagosome maturation, we investigated how perturbing the GTPase cycles of Rab5 and Rab7, GTPases that regulate early and late endosome fusion, respectively, would affect phagosome maturation. Retroviral transduction of the constitutively activated forms of both GTPases into primary murine macrophages had no effect on Mycobacterium avium retention in an early endosomal compartment. Interestingly, expression of dominant negative Rab5, Rab5(S34N), but not dominant negative Rab7, resulted in a significant increase in colocalization of M. avium with markers of late endosomes/lysosomes and increased mycobacterial killing. This colocalization was specific to mycobacteria since Rab5(S34N) expressing cells showed diminished trafficking of endocytic tracers to lysosomes. We further demonstrated that maturation of M. avium phagosomes was halted in Rab5(S34N) expressing macrophages supplemented with exogenous iron. These findings suggest that fusion with early endosomes is required for mycobacterial retention in early phagosomal compartments and that an inadequate supply of iron is one factor in mycobacteria's inability to prevent the normal maturation process in Rab5(S34N)-expressing macrophages.
Figures
Figure 1.
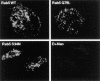
HA-Rab5 expression in transduced murine macrophages. BMMs were transduced with HA-tagged Rab5 WT (A), Rab5(Q79L) (B), or Rab5(S34N) (C). The expression and distribution of Rab5 in transduced BMMs was determined by confocal immunofluorescence microscopy using a mAb specific to the HA tag. Rab5-transduced BMMs show a characteristic punctate pattern. Background levels of HA staining were determined by using the anti-HA antibody on BMMs transduced with untagged Ev-Neo (D) and visualizing the macrophages under conditions identical to those used with the HA-Rab–transduced BMMs. Image bar, 16 μm. Confocal sections, 0.5 μm.
Figure 2.
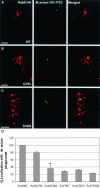
Colocalization of M. avium 101 with Rab5 but not Rab7 in transduced BMMs. BMMs were infected with M. avium 101 and with retrovirus encoding for HA-tagged Rab5 WT (A), Rab5(Q79L) (B), and Rab5(S34N) (C). Transduced macrophages were labeled for HA-tagged Rab proteins (red), and colocalization with the FITC-labeled bacilli (green) was determined by confocal immunofluorescence microscopy. Coincident staining appears yellow in the merged images. The number of mycobacterial phagosomes (n = 50 per experiment) that colocalize with the HA-Rab proteins were determined and expressed as the mean ± SD for three separate experiments (D). Significant differences were observed between Rab5(S34N) and Rab5 WT in their colocalization with the M. avium phagosome. *p < 0.0001. Image bar, 16 μm. Confocal sections, 0.5 μm.
Figure 3.
Trafficking of M. avium 101 to a LAMP1-positive, TR-negative compartment in macrophages expressing Rab5(S34N). BMMs expressing HA-tagged Rab5 WT (A and D), Rab5(Q79L) (B and E), or Rab5(S34N) (C and F) were stained for TR (red, A–C) or LAMP1 (red,
d
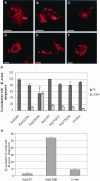
–F), and the amount of colocalization with M. avium 101 (green) was determined by confocal immunofluorescence microscopy. Coincident staining appears yellow in the merged images. The number of mycobacterial phagosomes (n = 50 per experiment) that stained positive for TR or LAMP1 (G) or LAMP2 (H) was quantified. Similar TR and LAMP1 staining experiments were completed using Rab7-transduced BMMs, and the results were quantified (G). The data are presented as the mean ± SD from three separate experiments. Comparison between Rab5(S34N)- and Rab5 WT–expressing macrophages indicate significant differences in the colocalization between the mycobacterial phagosome and endosome/lysosome markers. (a) p = 0.0348, (b) p < 0.0001. Image bar, 16 μm. Confocal sections, 0.5 μm.
Figure 4.
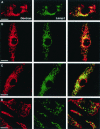
Minimal colocalization between dextran and LAMP1 in Rab5(S34N)-expressing macrophages. BMMs were infected with retrovirus containing the pLZRS constructs: Ev-Neo (A), Rab5 WT (B), Rab5(Q79L) (C), and Rab5(S34N) (D). Transduced BMMs were incubated with 1.5 μg of Texas-Red–labeled dextran for 5 min, washed, and incubated for an additional 30 min with fresh BMM media to chase the dextran to the lysosome. BMMs were then fixed, permeabilized, and stained for LAMP1 (green). Coincident staining appears yellow in the merged images. Data shown is representative of three separate experiments. Image bar, 16 μm. Confocal sections, 0.5 μm.
Figure 5.
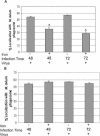
The addition of Fe-citrate to Rab5(S34N)-expressing macrophages significantly decreases the number of LAMP1-positive M. avium 101 phagosomes. The M. avium 101-infected BMMs expressing the Rab5(S34N) mutant were treated with 100 μM Fe-citrate (A) or with 100 μM of iron complexed to transferrin (B) for either 48 or 72 h before fixing and staining the macrophages for LAMP1. As controls some Rab5(S34N)-expressing BMMs infected with M. avium 101 were left untreated. The degree of colocalization between the mycobacterial phagosome and LAMP1 was determined as described in Figure 3. The data are presented as the mean ± SD from three separate experiments. Comparison between iron-treated and untreated transduced macrophages. (a) p = 0.0022, (b) p = 0.0006.
Figure 6.
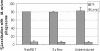
Addition of Fe-citrate to macrophages transduced with Rab5 WT or Ev-Neo had no effect on M. avium trafficking. BMMs infected with M. avium 101 were left untreated or retrovirally transduced with the Rab5 WT or Ev-Neo pLZRS constructs. The infected BMMs were incubated with Fe-citrate as described in Figure 5. The cells were fixed, permeabilized, and stained for either LAMP1 or TR. The number of mycobacterial phagosomes (n = 50 per experiment) that colocalize with LAMP1 or TR was determined and expressed as the mean ± SD for three separate experiments.
Figure 7.
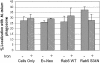
Addition of Fe-citrate had no effect on the colocalization of LAMP1 with phagosomes containing heat-killed M. avium in macrophages expressing Rab5(S34N). BMMs infected with heat-killed M. avium 101 were left untreated or retrovirally transduced with Rab5 WT, Rab5(S34N), or Ev-Neo pLZRS. The infected BMMs were incubated with Fe-citrate for 48 h as described in Figure 5. The fixed and permeabilized cells were stained for LAMP1. The number of mycobacterial phagosomes that colocalized with LAMP1 were quantitated and expressed as the mean ± SD from three separate experiments.
Figure 8.
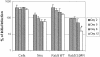
Killing of M. avium 101 in macrophages expressing Rab5(S34N). Triplicate wells of BMMs were infected with M. avium and either left untreated or retrovirally transduced with the Rab5 WT, Rab5(S34N), or Ev-Neo pLZRS constructs. One set of infected BMMs were harvested immediately after the viral transduction and this served as the initial infection load (i.e., 100%). Infected BMMs were also harvested after 2, 5, 8, and 12 d. Bacilli from lysed BMMs were quantitated by serial dilution and expressed, for each set of transductions, as percent of initial infection load ±SD. Initial infection loads were: 3.95 × 106 for nontransduced cells, 7.44 × 106 for Ev-Neo, 3.68 × 106 for Rab5 WT, and 1.13 × 107 for Rab5(S34N)-transduced BMMs. A representative of two experiments is presented. *p = 0.035.
Similar articles
- Modulation of Rab5 and Rab7 recruitment to phagosomes by phosphatidylinositol 3-kinase.
Vieira OV, Bucci C, Harrison RE, Trimble WS, Lanzetti L, Gruenberg J, Schreiber AD, Stahl PD, Grinstein S. Vieira OV, et al. Mol Cell Biol. 2003 Apr;23(7):2501-14. doi: 10.1128/MCB.23.7.2501-2514.2003. Mol Cell Biol. 2003. PMID: 12640132 Free PMC article. - Effects of cytokines on mycobacterial phagosome maturation.
Via LE, Fratti RA, McFalone M, Pagan-Ramos E, Deretic D, Deretic V. Via LE, et al. J Cell Sci. 1998 Apr;111 ( Pt 7):897-905. doi: 10.1242/jcs.111.7.897. J Cell Sci. 1998. PMID: 9490634 - Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest.
Fratti RA, Backer JM, Gruenberg J, Corvera S, Deretic V. Fratti RA, et al. J Cell Biol. 2001 Aug 6;154(3):631-44. doi: 10.1083/jcb.200106049. J Cell Biol. 2001. PMID: 11489920 Free PMC article. - Mycobacterial phagosome maturation, rab proteins, and intracellular trafficking.
Deretic V, Via LE, Fratti RA, Deretic D. Deretic V, et al. Electrophoresis. 1997 Dec;18(14):2542-7. doi: 10.1002/elps.1150181409. Electrophoresis. 1997. PMID: 9527483 Review. - Who's in control? Principles of Rab GTPase activation in endolysosomal membrane trafficking and beyond.
Borchers AC, Langemeyer L, Ungermann C. Borchers AC, et al. J Cell Biol. 2021 Sep 6;220(9):e202105120. doi: 10.1083/jcb.202105120. Epub 2021 Aug 12. J Cell Biol. 2021. PMID: 34383013 Free PMC article. Review.
Cited by
- Escape from the Phagosome: The Explanation for MHC-I Processing of Mycobacterial Antigens?
Harriff MJ, Purdy GE, Lewinsohn DM. Harriff MJ, et al. Front Immunol. 2012 Mar 5;3:40. doi: 10.3389/fimmu.2012.00040. eCollection 2012. Front Immunol. 2012. PMID: 22566923 Free PMC article. - Host innate immune response to Mycobacterium tuberculosis.
Bhatt K, Salgame P. Bhatt K, et al. J Clin Immunol. 2007 Jul;27(4):347-62. doi: 10.1007/s10875-007-9084-0. Epub 2007 Mar 16. J Clin Immunol. 2007. PMID: 17364232 Review. - MAV_4644 Interaction with the Host Cathepsin Z Protects Mycobacterium avium subsp. hominissuis from Rapid Macrophage Killing.
Lewis MS, Danelishvili L, Rose SJ, Bermudez LE. Lewis MS, et al. Microorganisms. 2019 May 21;7(5):144. doi: 10.3390/microorganisms7050144. Microorganisms. 2019. PMID: 31117286 Free PMC article. - Analysis of phagosomal proteomes: from latex-bead to bacterial phagosomes.
Li Q, Jagannath C, Rao PK, Singh CR, Lostumbo G. Li Q, et al. Proteomics. 2010 Nov;10(22):4098-116. doi: 10.1002/pmic.201000210. Proteomics. 2010. PMID: 21080496 Free PMC article. Review. - Gallium nanoparticles facilitate phagosome maturation and inhibit growth of virulent Mycobacterium tuberculosis in macrophages.
Choi SR, Britigan BE, Moran DM, Narayanasamy P. Choi SR, et al. PLoS One. 2017 May 18;12(5):e0177987. doi: 10.1371/journal.pone.0177987. eCollection 2017. PLoS One. 2017. PMID: 28542623 Free PMC article.
References
- Baker, E., Baker, S.M., and Morgan, E.H. (1998). Characterisation of non-transferrin-bound iron (ferric citrate) uptake by rat hepatocytes in culture. Biochim. Biophys. Acta 1380, 21–30. - PubMed
- Barbieri, M.A., Li, G., Colombo, M.I., and Stahl, P.D. (1994). Rab5, an early acting endosomal GTPase, supports in vitro endosome fusion without GTP hydrolysis. J. Biol. Chem. 269, 18720–18722. - PubMed
- Bellamy, R. (1999). The natural resistance-associated macrophage protein and susceptibility to intracellular pathogens. Microbes Infect. 1, 23–27. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical