Synaptopodin-deficient mice lack a spine apparatus and show deficits in synaptic plasticity - PubMed (original) (raw)
. 2003 Sep 2;100(18):10494-9.
doi: 10.1073/pnas.1832384100. Epub 2003 Aug 19.
Martin Korte, Sophie Chabanis, Alexander Drakew, Herbert Schwegler, Giulia Good Stefani, Aimee Zuniga, Karin Schwarz, Tobias Bonhoeffer, Rolf Zeller, Michael Frotscher, Peter Mundel
Affiliations
- PMID: 12928494
- PMCID: PMC193589
- DOI: 10.1073/pnas.1832384100
Synaptopodin-deficient mice lack a spine apparatus and show deficits in synaptic plasticity
Thomas Deller et al. Proc Natl Acad Sci U S A. 2003.
Abstract
The spine apparatus is a cellular organelle that is present in many dendritic spines of excitatory neurons in the mammalian forebrain. Despite its discovery >40 years ago, the function of the spine apparatus is still unknown although calcium buffering functions as well as roles in synaptic plasticity have been proposed. We have recently shown that the 100-kDa protein synaptopodin is associated with the spine apparatus. Here, we now report that mice homozygous for a targeted deletion of the synaptopodin gene completely lack spine apparatuses. Interestingly, this absence of the spine apparatus is accompanied by a reduction in hippocampal long-term potentiation (LTP) in the CA1 region of the hippocampus and by an impairment of spatial learning in the radial arm maze test. This genetic analysis points to a role of the spine apparatus in synaptic plasticity.
Figures
Fig. 1.
Generation of _synaptopodin_-deficient mice. (A) Gene targeting strategy. The ORF of the synaptopodin gene (amino acids 2-690; black box) was replaced in frame by a lacZ cassette by using homologous recombination. (B) Southern blot analysis of genomic DNA from wild type (+/+) and two independent, heterozygous (+/-) ES cell clones. The correct recombination was determined by using a 5′ external probe on _Eco_RI/_Bg_III digests and a 3′ internal probe on _Xba_I/_Nco_I digests, as well as Neomycin and LacZ probes (not shown). Both clones shown were used to generate chimeric mice. (C) Western blot analysis of cytosolic forebrain extracts prepared exactly as described (26) from (+/+), (+/-), and (-/-) mice by using the Synaptopodin-specific rabbit polyclonal antibody NT (26). The 100-kDa band corresponding to Synaptopodin was strongly expressed in +/+ and weaker in +/- mice, but was absent from -/- mice.
Fig. 2.
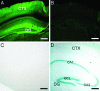
Distribution of Synaptopodin protein and transcripts. (A) Immunofluorescence microscopy shows the expression of Synaptopodin in wild-type neocortex (CTX) and hippocampus. Note that immunolabeling is strongest in the dendritic layers (27, 28), particularly in those of the dentate gyrus (DG). (B) Absence of immunofluorescence in the mutant confirms the specificity of Synaptopodin immunolabeling and the successful deletion of the synaptopodin gene. (C) Absence of β-galactosidase activity in wild type. (D) Synaptopodin mRNA is expressed in the granule cell layer (GCL) of the dentate gyrus (DG), pyramidal cell layer of hippocampal areas CA3 and CA1 (27, 28), and in various layers of the neocortex (CTX) as shown by β-galactosidase activity in the mutant. (Scale bars: 400 μm.)
Fig. 3.
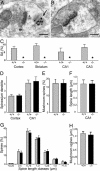
_Synaptopodin_-deficient mice lack a spine apparatus. (A) Spine apparatus (arrow) in a wild-type hippocampal neuron; immunolabeled for Synaptopodin protein (1.4 nm gold grains, silver-intensified). (Scale bar: 0.2 μm.) (B) Absence of spine apparatus (and of Synaptopodin protein) in a hippocampal neuron from a _synaptopodin_-deficient mouse. (Scale bar: 0.2 μm.) (C) Average percentage (plus SD) of spines with a spine apparatus (SA) in wild-type (+/+) animals (n = 5) and _synaptopodin_-deficient (-/-) mice (n = 5). Number of spines analyzed (+/+ versus -/-): cortex (2,396/1,889), striatum (2,218/1,985), hippocampal area CA1 (2,488/2,218), hippocampal area CA3 (1,742/1,635). In _synaptopodin_-deficient mice (asterisks), the spine apparatus is absent in all regions analyzed. (D) The number of spines (plus SEM) on apical dendrites of Golgi-impregnated layer 5 pyramidal neurons and on dendrites of CA1 pyramidal neurons is similar in wild-type (+/+; n = 5) and _synaptopodin_-deficient (-/-; n = 5) mice. (E) The percentage of mushroom spines (plus SD) is comparable in wild type (+/+; n = 5) and _synaptopodin_-deficient mice (-/-; n = 5). (F) The length of spines (plus SD) was measured on dendrites of CA1 pyramidal neurons. No significant difference was observed between wild-type (+/+; n = 5) and _synaptopodin_-deficient (-/-; n = 5) mice. (G) Analysis of spine length classes (each class: 0.25 μm; plus SD) revealed a comparable distribution of spines in wild-type (+/+; n = 5) and _synaptopodin_-deficient (-/-; n = 5) mice. (H) The selective analysis of mushroom spine length (plus SD) did not show a significant difference between wild-type (+/+; n = 5) and _synaptopodin_-deficient (-/-; n = 5) mice.
Fig. 4.
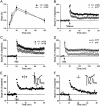
Reduced LTP in the hippocampus of _synaptopodin_-deficient mice. (A) Paired-pulse facilitation (PPF) was not significantly different between mutant and wild-type mice. The percentages denote the ratio of the second EPSP slope size to the first EPSP slope. PPF was tested for 10-, 20-, 40-, 80-, and 160-ms interstimulus intervals (ISI). (B) Group data for fEPSP recordings before and after tetanus (100 Hz) application. The difference between synaptopodin mutant and wild-type mice is significant (P < 0.01). Error bars, SEM; n, number of slices. (C) Group data for fEPSP recordings before and after theta burst (TBS; 100 Hz) application. Also for TBS application the difference between mutant and wild-type mice is significant (P < 0.01; t test, two-sided). Error bars, SEM; n, number of slices. (D) Group data for fEPSP recordings before and 3 h after TBS (100 Hz) application. L-LTP is also affected in _synaptopodin_-deficient mice. The difference between mutant and wild-type mice is significant (P < 0.05; t test). Error bars, SEM; n, number of slices. Only slices that showed E-LTP were included in the analysis. (E and F) Single experiment for a wild-type mouse (E) and a _synaptopodin_-deficient animal (F). Arrow, application of TBS (100 Hz). Sample fEPSP traces before and after TBS application are displayed in Insets. Small letters next to the curve in the main graph indicate the time points at which the sample responses were taken.
Fig. 5.
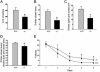
Learning defects in _synaptopodin_-deficient mice. (A) Locomotor activity in the open field. Bars indicate mean numbers (+SEM) of line crossings. Mutant mice (n = 9) show less locomotor activity than wild-type controls (n = 10). (_B_-D) Anxiety-related behavior in the elevated plus maze. Frequency of closed (B) and all (C) arm entries (mean + SEM). The ratio of entries into closed arms versus all arms is illustrated in D. Note that mutant mice (n = 9) are less anxious than wild-type controls (n = 10), and that this difference is not caused by reduced locomotor activity. (E) Spatial learning in the radial arm maze. Mean numbers (±SEM) of spatial working memory errors of wild-type (n = 16; dots) and mutant (n = 14; triangles) mice during the 5-day training period. From day three on, mutant mice show significantly more failures than wild-type mice (*, P < 0.05).
Similar articles
- Stabilization of Spine Synaptopodin by mGluR1 Is Required for mGluR-LTD.
Speranza L, Inglebert Y, De Sanctis C, Wu PY, Kalinowska M, McKinney RA, Francesconi A. Speranza L, et al. J Neurosci. 2022 Mar 2;42(9):1666-1678. doi: 10.1523/JNEUROSCI.1466-21.2022. Epub 2022 Jan 19. J Neurosci. 2022. PMID: 35046120 Free PMC article. - A role for synaptopodin and the spine apparatus in hippocampal synaptic plasticity.
Deller T, Bas Orth C, Del Turco D, Vlachos A, Burbach GJ, Drakew A, Chabanis S, Korte M, Schwegler H, Haas CA, Frotscher M. Deller T, et al. Ann Anat. 2007;189(1):5-16. doi: 10.1016/j.aanat.2006.06.013. Ann Anat. 2007. PMID: 17319604 Review. - The spine apparatus, synaptopodin, and dendritic spine plasticity.
Segal M, Vlachos A, Korkotian E. Segal M, et al. Neuroscientist. 2010 Apr;16(2):125-31. doi: 10.1177/1073858409355829. Neuroscientist. 2010. PMID: 20400711 Review. - Impairment of in vivo theta-burst long-term potentiation and network excitability in the dentate gyrus of synaptopodin-deficient mice lacking the spine apparatus and the cisternal organelle.
Jedlicka P, Schwarzacher SW, Winkels R, Kienzler F, Frotscher M, Bramham CR, Schultz C, Bas Orth C, Deller T. Jedlicka P, et al. Hippocampus. 2009 Feb;19(2):130-40. doi: 10.1002/hipo.20489. Hippocampus. 2009. PMID: 18767067 - Essential role for synaptopodin in dendritic spine plasticity of the developing hippocampus.
Zhang XL, Pöschel B, Faul C, Upreti C, Stanton PK, Mundel P. Zhang XL, et al. J Neurosci. 2013 Jul 24;33(30):12510-8. doi: 10.1523/JNEUROSCI.2983-12.2013. J Neurosci. 2013. PMID: 23884954 Free PMC article.
Cited by
- Exploring the O-GlcNAc proteome: direct identification of O-GlcNAc-modified proteins from the brain.
Khidekel N, Ficarro SB, Peters EC, Hsieh-Wilson LC. Khidekel N, et al. Proc Natl Acad Sci U S A. 2004 Sep 7;101(36):13132-7. doi: 10.1073/pnas.0403471101. Epub 2004 Aug 30. Proc Natl Acad Sci U S A. 2004. PMID: 15340146 Free PMC article. - Synaptopodin regulates the actin-bundling activity of alpha-actinin in an isoform-specific manner.
Asanuma K, Kim K, Oh J, Giardino L, Chabanis S, Faul C, Reiser J, Mundel P. Asanuma K, et al. J Clin Invest. 2005 May;115(5):1188-98. doi: 10.1172/JCI23371. Epub 2005 Apr 1. J Clin Invest. 2005. PMID: 15841212 Free PMC article. - The Use of Whole Exome Sequencing in a Cohort of Transgender Individuals to Identify Rare Genetic Variants.
Theisen JG, Sundaram V, Filchak MS, Chorich LP, Sullivan ME, Knight J, Kim HG, Layman LC. Theisen JG, et al. Sci Rep. 2019 Dec 27;9(1):20099. doi: 10.1038/s41598-019-53500-y. Sci Rep. 2019. PMID: 31882810 Free PMC article. - Disease predictability review using common biomarkers appearing in diabetic nephropathy and neurodegeneration of experimental animals.
Yi SS. Yi SS. Lab Anim Res. 2022 Feb 7;38(1):3. doi: 10.1186/s42826-022-00113-8. Lab Anim Res. 2022. PMID: 35130988 Free PMC article. Review. - Stabilization of Spine Synaptopodin by mGluR1 Is Required for mGluR-LTD.
Speranza L, Inglebert Y, De Sanctis C, Wu PY, Kalinowska M, McKinney RA, Francesconi A. Speranza L, et al. J Neurosci. 2022 Mar 2;42(9):1666-1678. doi: 10.1523/JNEUROSCI.1466-21.2022. Epub 2022 Jan 19. J Neurosci. 2022. PMID: 35046120 Free PMC article.
References
- Fischer, M., Kaech, S., Knutti, D. & Matus, A. (1998) Neuron 20, 847-854. - PubMed
- Yuste, R. & Denk, W. (1995) Nature 375, 682-684. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous